- CK Fox et al. NEJM 2024; https://www.nejm.org/doi/full/10.1056/NEJMoa2407379. Liraglutide for Children 6 to <12 Years of Age with Obesity — A Randomized Trial
- T Barrett et al. NEJM 2024 (editorial);/doi/full/10.1056/NEJMe2410560. Childhood Obesity and GLP-1 Receptor Agonists — A Coming of Age?
Background: The glucagon-like peptide-1 (GLP-1) analogues liraglutide and semaglutide are approved by the Food and Drug Administration and the European Medicines Agency for long-term weight management in adolescents 12 years of age or older with obesity, as adjunct treatments to lifestyle interventions.14-19 These medications act centrally to increase satiety signaling, reduce appetite and energy intake, and decrease food reward; these medications also increase postprandial insulin levels, reduce glucagon secretion, and delay gastric emptying.
Methods: this phase 3a (SCALE kids) trial, which consisted of a 56-week treatment period and a 26-week follow-up period, we randomly assigned children (n=82) (6 to <12 years of age) with obesity, in a 2:1 ratio, to receive either once-daily subcutaneous liraglutide at a dose of 3.0 mg (or the maximum tolerated dose) or placebo, plus lifestyle interventions.
Key findings:
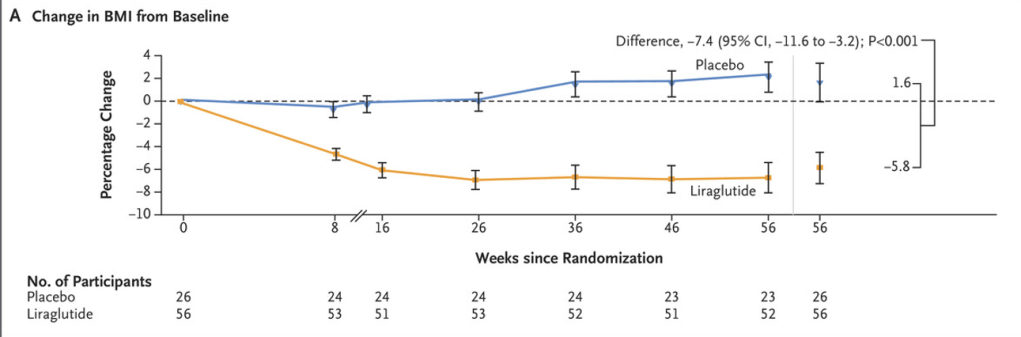
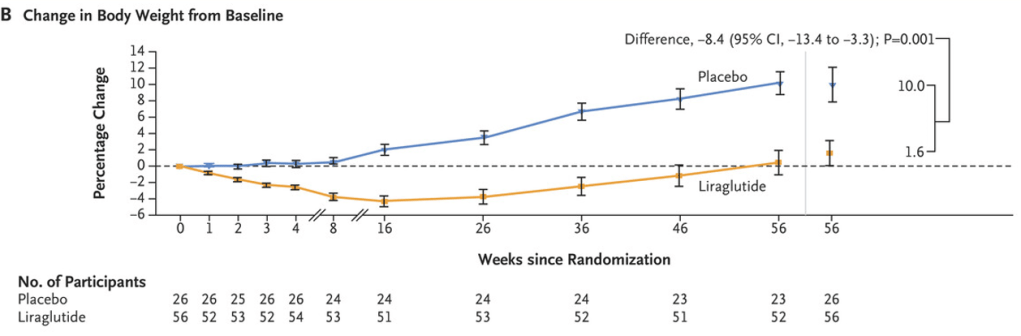
- At week 56, the mean percentage change from baseline in BMI was −5.8% with liraglutide and 1.6% with placebo
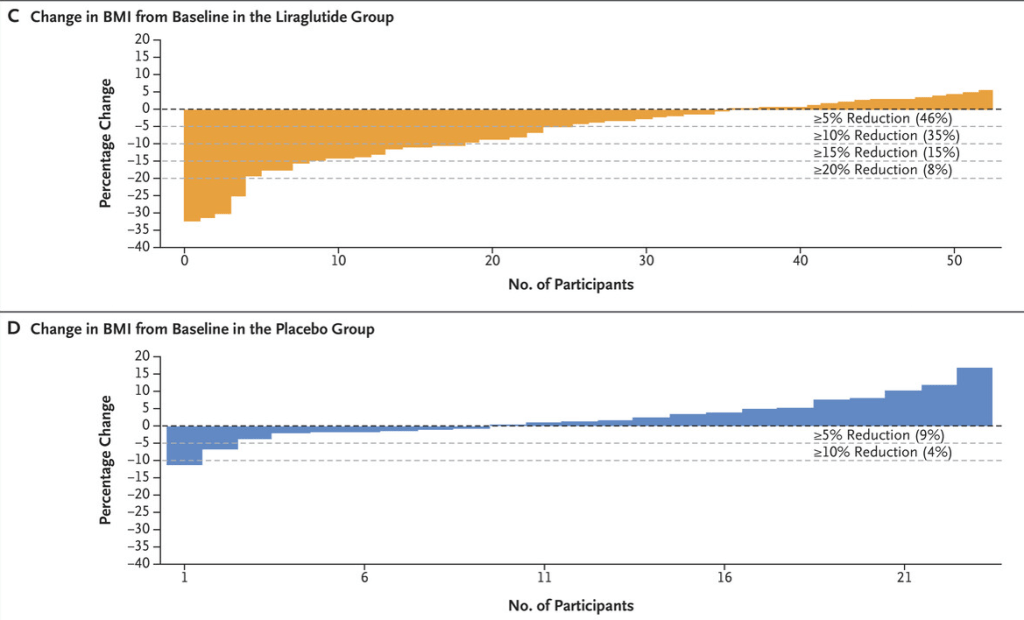
- A reduction in BMI of at least 5% occurred in 46% of participants in the liraglutide group and in 9% of participants in the placebo group (adjusted odds ratio, 6.3)
- The most common adverse events were gastrointestinal disorders, which were reported in 45 of 56 participants (80%) in the liraglutide group and in 14 of 26 participants (54%) in the placebo group. Three cases of vomiting in the liraglutide group were considered by investigators to be serious (each required emergency care); however, none of the events required hospitalization and all resolved without sequelae.
- In the treated group, improvements were also observed in diastolic blood pressure and the glycated hemoglobin level
- The editorial: “Fox et al. provide much-needed evidence for the effects of a GLP-1 receptor agonist in young children with obesity, offering a therapeutic option in prepubertal children with severe obesity as an adjunct to healthy lifestyle interventions.”
My take: It is good that there are now effective therapeutic pharmaceutical options for obesity, especially for those developing complications. Long term studies are needed as the effects of these medications on weight are not sustained in those who stop them. Given the need for indefinite therapy, other public health measures are needed to try to reverse the high prevalence of obesity.
Related blog posts:
- Primary Prevention of Obesity Still Needed
- Meds for Obesity: AAP Guidelines
- Semaglutide in Adolescent Obesity
- AGA Guidelines for Adults with Obesity
- What Really Causes Obesity and Weight Bias
- Pharmacotherapy for Obesity
- Good Review on Newest Medications for Obesity
- Getting over the Stigma of Medicines for Anxiety/Depression and Obesity