MC Choy et al. The Lancet Gastroenterology 2024; Intensified versus standard dose infliximab induction therapy for steroid-refractory acute severe ulcerative colitis (PREDICT-UC): an open-label, multicentre, randomised controlled trial
Methods: In this open-label, multicenter, randomized controlled trial, patients aged 18 years or older from 13 Australian tertiary hospitals with intravenous steroid-refractory ASUC were randomly assigned (1:2) to receive a first dose of 10 mg/kg infliximab or 5 mg/kg infliximab (randomization 1). Block randomization was used and stratified by history of thiopurine exposure and study site, with allocation concealment maintained via computer-generated randomization. Patients in the 10 mg/kg group (intensified induction strategy [IIS]) received a second dose at day 7 or earlier at the time of non-response; all patients in the 5 mg/kg group were re-randomized between day 3 and day 7 (1:1; randomization 2) to a standard induction strategy (SIS) or accelerated induction strategy (AIS), resulting in three induction groups. Patients in the SIS group received 5 mg/kg infliximab at weeks 0, 2, and 6, with an extra 5 mg/kg dose between day 3 and day 7 if no response. Patients in the AIS group received
5 mg/kg infliximab at weeks 0, 1, and 3, with the week 1 dose increased to 10 mg/kg and given between day 3 and day 7 if no response.
Thus, this was the first RCT comparing an intensified induction strategy (IIS; 10 mg/kg infliximab at weeks 0 and 1, with the second dose given earlier if no clinical response), an accelerated induction strategy (AIS; 5 mg/kg infliximab at weeks 0, 1, and 3, with the second dose increased to 10 mg/kg and given earlier if no response), and a standard induction strategy (SIS; 5 mg/kg at weeks 0, 2 and 6; with an extra 5 mg/kg dose before day 7 if no
response) in steroid-refractory patients with ASUC.
Key findings:
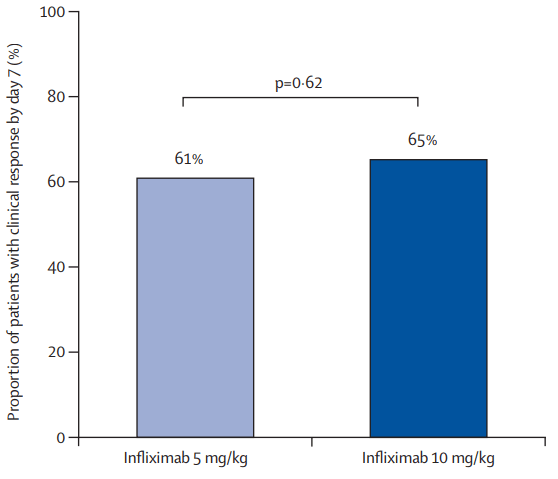
- There was no significant difference in the proportion of patients who had a clinical response by day 7 between the 10 mg/kg and 5 mg/kg groups: 65% vs 61%
- In patients with a baseline albumin of less than 25 g/L, a day 7 response occurred in nine (64%) of 14 patients in the 10 mg/kg group versus 14 (45%) of 31 in the 5 mg/kg group (RR 1·43, p=0·17)
- In patients with a baseline CRP of 50 mg/L or higher, a day 7 response occurred in six (60%) of ten patients in the 10 mg/kg group versus eight (42%) of 19 in the 5 mg/kg group (RR 1·39, p=0·34)
- The proportions of patients with clinical response at day 14: 74% in the IIS group, 73% in the AIS group, and 68% of 44 in the SIS group.
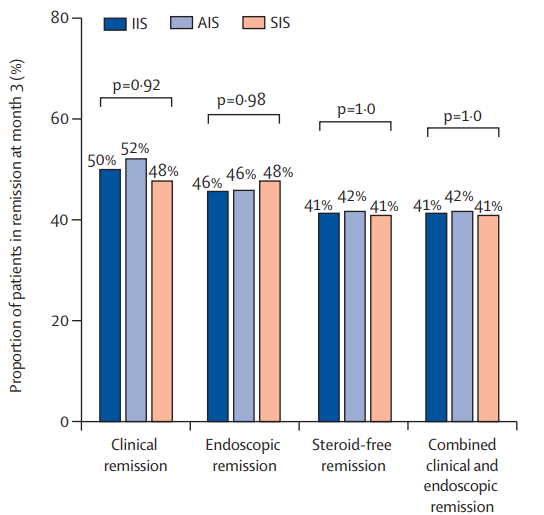
- The clinical remission at month 3: 50% in the IIS group, 52% in the AIS group, and 48% in the SIS group
- The steroid-free remission at month 3: 41% in the IIS group, 42% in the AIS group, and 41% in the SIS group
- The endoscopic remission at month 3: 46% in the IIS group, 46% in the AIS group, and 48% in the SIS group
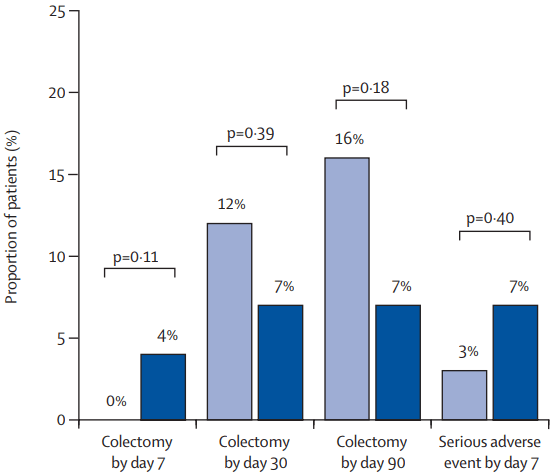
- The colectomy rate at month 3: 7% in the IIS group, 19% in the AIS group, and 12% in the SIS group colectomy at month 3 were not significantly different between group (P=0.20)
- The colectomy rate at month 12: 7% in the IIS group, 22% in the AIS group, and 15% in the SIS group colectomy at month 3 were not significantly different between group (p=0.13)
- In post-hoc analysis of second-dose salvage strategies (among the group who had not responded at day7), a clinical response was observed in 19 (59%) of 32 patients who received a 10 mg/kg salvage dose versus nine (64%) of 14 who received a 5 mg/kg salvage dose (RR 0·92). Endoscopic remission at month 3 was observed in 11 (34%) who received 10 mg/kg salvage versus six (43%) who received 5 mg/kg salvage (RR 0·80). Colectomy by 3 months occurred in ten (31%) who received 10 mg/kg salvage compared with three (21%) who received 5 mg/kg salvage (HR 1·46)
- Higher proportions of patients with clinical and biochemical remission between weeks 2 and 6 were apparent in the IIS and AIS groups versus the SIS group, but by 3 months, these differences were lost
My take: Intensified, accelerated, and standard induction regimens in the PREDICT-UC study did not result in a statistically-significant difference in clinical response by day 14 or in remission or colectomy rates by month 3. However, there are some important caveats:
- There appeared to be a trend towards a lower colectomy rate in the IIS group.
- There appeared to be a favorable trend towards an improved response to IIS group in those with low albumin (<25 g/L) and high CRP (>5 mg/L). The smaller numbers in these subgroups could have precluded statistical significance
- Also, even the SIS group were able to receive a 4th induction 5 mg/kg dose between days 3-7 if they had not responded to treatment
- In patients who had not responded to either 10 mg/kg or 5 mg/kg, a salvage dose at day 7 resulted in a >60% response rate
- It is possible that a sustained strategy of more aggressive dosing (not done in this study) aided with therapeutic drug monitoring could result in better outcomes following IIS
