N Mathieu et al. Clin Gastroenterol Hepatol 2025; 23: 2597 – 2606. Open Access! PErsistence and Safety of Subcutaneous Infliximab 1 Year After Switch From Intravenous Route in IBD Patients in REMission
Methods: The PEREM (PErsistence, effectiveness and safety of subcutaneous infliximab after switch from intravenous infliximab in IBD patients in REMission) study, a prospective national French cohort trial, enrolled 426 patients with IBD. Participants were in steroid-free clinical remission for at least 6 months on IV-IFX when they switched to SC-IFX. 56% were on IV-IFX standard dosing (5 mg/kg 8-weekly) and 16% received combination therapy with an immunomodulator drug at baseline. All patients were switched to SC-IFX standard dosing (ie, 120 mg every other week). The treatment could be intensified during follow-up, either to 120 mg every week or 240 mg every other week.
Key Findings:
- At week 48, SC-IFX persistence was 95.4%
- 86.9% of patients were in steroid-free clinical remission
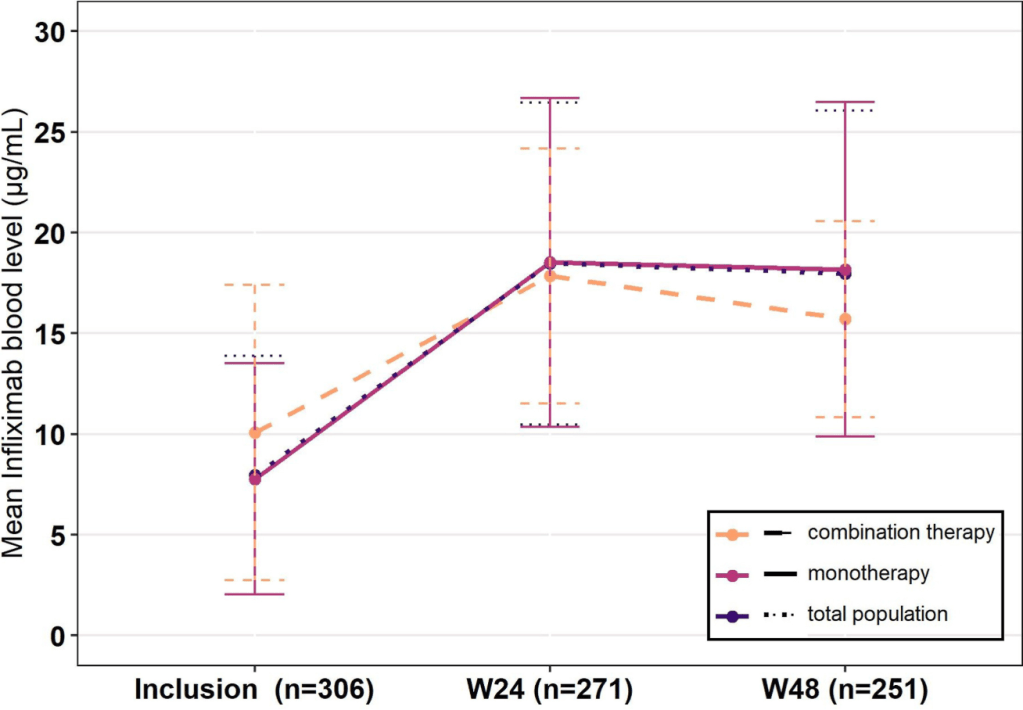
- Mean infliximab levels were 8.0 μg/mL at inclusion and 18.0 μg/mL at week 48 (P < .0001)
- Among the 19 (4.5%) patients who stopped SC-IFX, 6 (1.4%) switched back to IV-IFX
- 23 (5.4%) patients required SC-IFX dose escalation
- Dosing at 10 mg/kg/Q4W had 100% SC IFX persistence compared to 95% for 5 mg/kg/Q8W; however, at the 48 week followup, there were only 6 patients in the higher dose compared to 149 in the lower dose
- Ongoing use of combination therapy was not associated with better persistence. Though, only 7 patients were receiving combination therapy at the 48 week followup
From the discussion:
- “The high persistence observed in the PEREM study is partly explained by the long-term control of the disease by the time of switch, the median time since last flare being over 5 years before inclusion. Henceforth, the persistence observed here is in accordance with results on long term maintenance of IV-IFX, the yearly persistence of IV-IFX without intervention being 87%.”
- SC-IFX was associated with higher levels. However, this was expected and higher levels are needed with SC administration. The “different bioavailability of SC-IFX compared with IV-IFX is responsible for different goals of infliximab blood levels depending on its route. In particular, a level above 20 μg/mL has been associated with higher rates of remission20” with SC-IFX.
My take: This study shows that SC-IFX is a good option for patients in long-term remission. With SC-IFX therapy, more effort is needed to make sure patients are adherent with therapy and monitoring in order to achieve optimal outcomes.
Related blog posts:
- Infliximab Thresholds with Subcutaneous vs Intravenous Administration for Crohn’s Disease
- Vedolizumab and Infliximab: Expected Dosing When Switching From IV to SC Routes
- LIBERTY Trials for Subcutaneous Infliximab
- FDA-Approved Subcutaneous Infliximab (Zymfentra) Now Available
- REMSWITCH: Infliximab IV to SC Study
