M-F Yuen et al. NEJM 2022; 387; 1957-1968. Efficacy and Safety of Bepirovirsen in Chronic Hepatitis B Infection
This study is nicely summarized in a “quick take” video and also reviewed in an accompanying editorial by J Hoofnagle (pages: 1996-1998).
In this phase 2b, randomized, investigator-unblinded trial involving 457 participants with chronic HBV infection (1/2 receiving nucleotide analogue (NA) therapy), the authors evaluated bepirovirsen is an antisense oligonucleotide that targets all hepatitis B virus (HBV) messenger RNAs and acts to decrease levels of viral proteins.
Background: HBV infection affects 4% of worldwide population and has a prevalence of 0.3% in the U.S. Worldwide, HBV causes more than 1/2 million deaths each year.
Key finding:
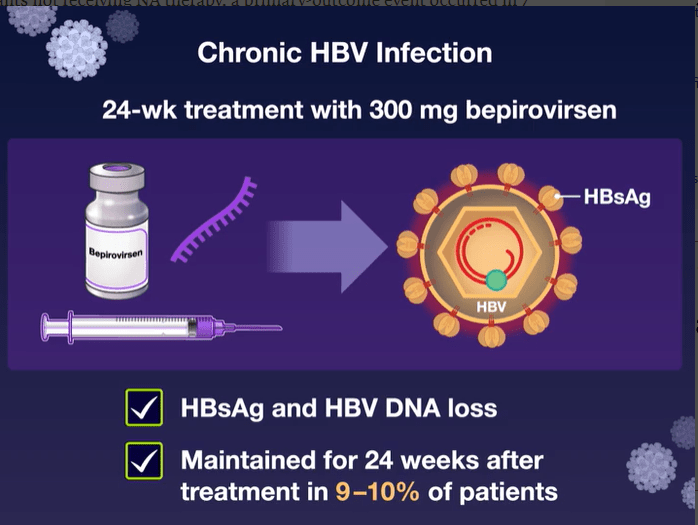
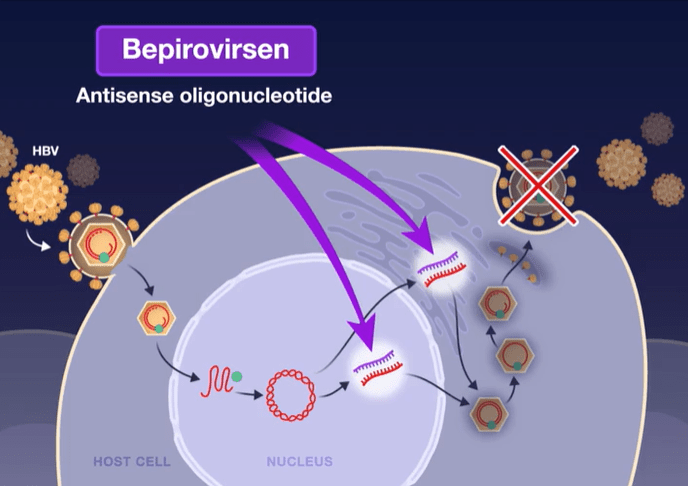
Mechanism of Action: Bepirovirsen is an antisense oligonucleotide that targets all hepatitis B virus (HBV) messenger RNAs and acts to decrease levels of viral proteins.
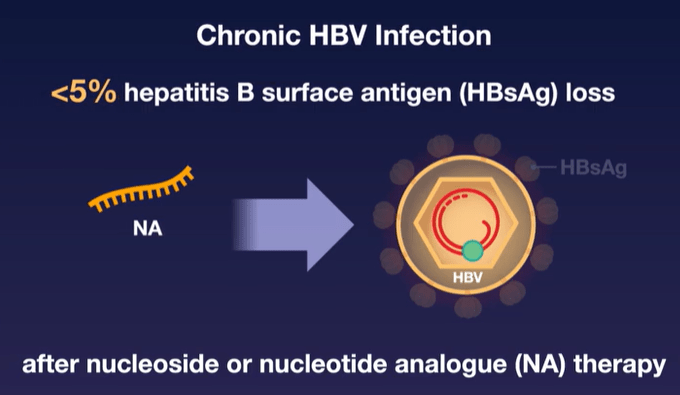
Current therapies (like entecavir and tenofovir) are able to suppress viral replication but have low rates of clearance of HBsAg and most often HBV relapses when medications are stopped. This is due to covalently closed circular DNA which can persist in hepatocytes despite these medications.

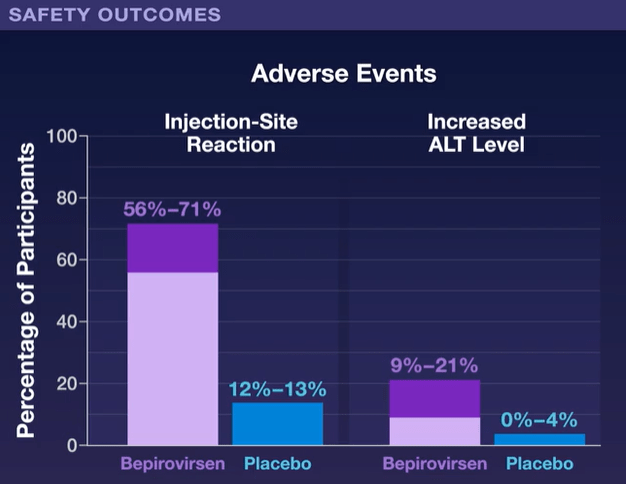

In Dr. Hoofnagle’s editorial, he notes that bepirovirsen is one of several RNA-based HBV therapies that are being pursued. There are also “the more malleable small interfering RNA molecules (“-sirans”) are currently in early-phase clinical trials.”
My take: While these studies point to new therapies for those afflicted with HBV infection, the best strategy for reducing HBV mortality and morbidity still relies of wide-scale use of the highly effective HBV vaccine.
