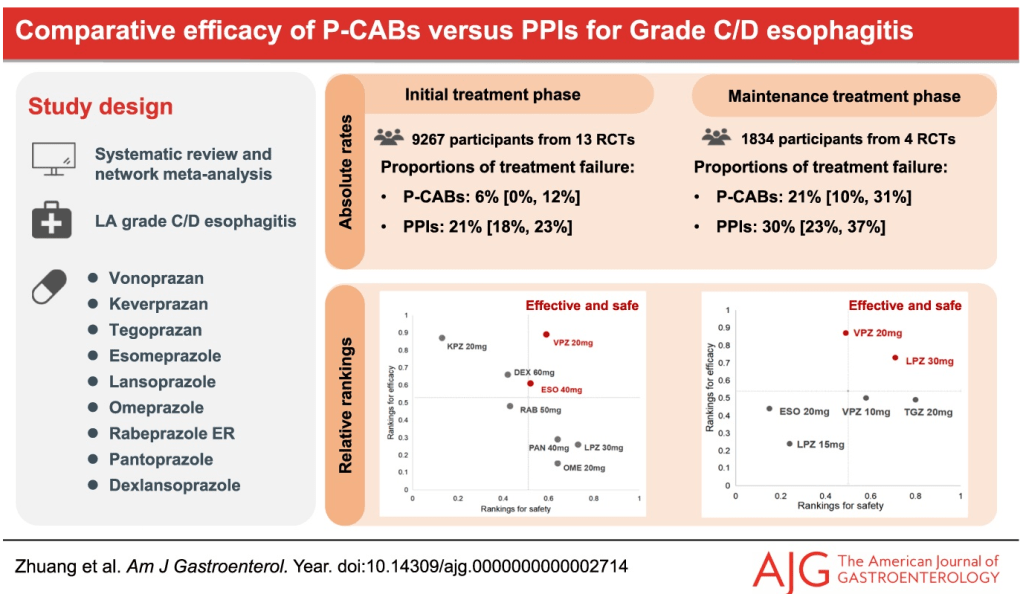
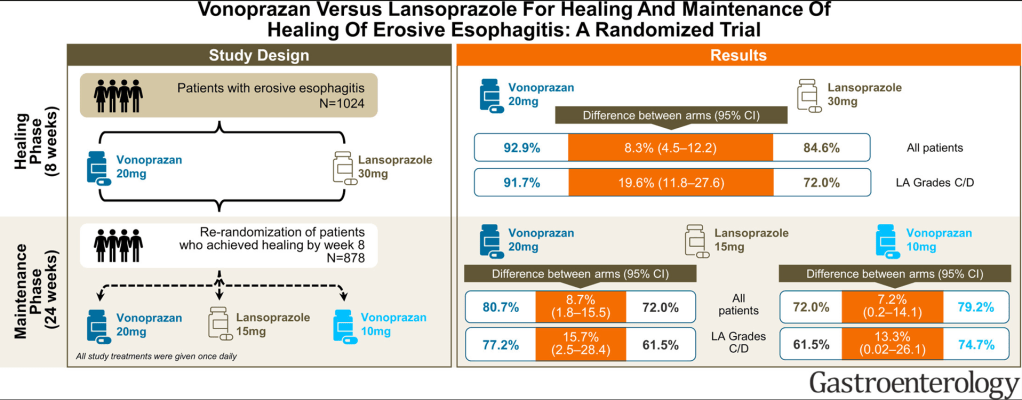
Thanks to Ben Gold for the reference in today’s post.
J-H Oh et al. Am J Gastroenterol 2025; DOI: 10.14309/ajg.0000000000002929 Randomized, Double-Blind, Active-Controlled Phase 3 Study to Evaluate Efficacy and Safety of Zastaprazan Compared With Esomeprazole in Erosive Esophagitis
Introduction: “Unlike PPIs, metabolism of zastaprazan in not dependent on CYP2C19, and it does not require enteric coating due to its acid stability. While PPIs bind irreversibly only to active proton pumps, zastaprazan can bind reversibly and competitively to both active and inactive proton pumps. Moreover, as prodrugs, PPIs necessitate activation into their active form within acidic conditions, typically requiring a regimen of 3-5 consecutive days of dosing…By contrast, P-CABs deliver a rapid onset of action and complete efficacy from the initial dose, as they can directly inhibit proton pumps.”
Methods: A phase III, multicenter, randomized, double-blind, noninferiority clinical study was conducted with 300 subjects (>19 yrs) with confirmed erosive esophagitis compared daily zastaprazan (20 mg) to esomeprazole (40 mg)
Key findings:
- The cumulative healing rate at week 8 were 97.92% (141/144) for zastaprazan and 94.93% (131/138) (P = 0.178) for esomeprazole.
- The healing rate at week 4 in the zastaprazan group was higher than the esomeprazole group (95.14% [137/144] vs 87.68% [121/138]; P = 0.026)
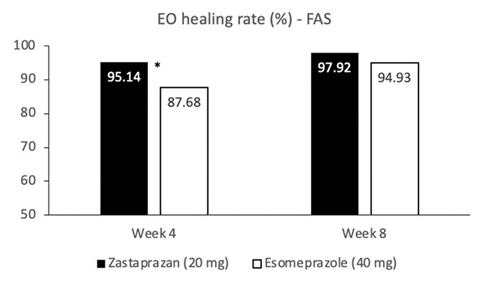
My take: Zastaprazan had higher healing rates at 4 weeks; results at 8 weeks were similar. This phase 3 study suggests that there will be other CABs besides vonoprazan approved for treating acid-related disorders.
Related blog posts:
- Practice Advice for Potassium-Competitive Acid Blockers
- Safety and Efficacy of Potassium Competitive Acid Blockers (3 Studies)
- Vonoprazan Treatment of Heartburn in Randomized Study of Patients with Non-Erosive Reflux Disease
- Is Vonoprazan Better Than Intravenous PPIs for High-Risk Peptic Ulcers?
- Why Vonoprazan Is More Effective For Erosive Esophagitis Than a Proton Pump Inhibitor
- Vonoprazan versus Lansoprazole for Initial Heartburn Relief