June 4, 2021: FDA Approves New Drug Treatment for Chronic Weight Management, First Since 2014
“The U.S. Food and Drug Administration approved Wegovy (semaglutide) injection (2.4 mg once weekly) for chronic weight management in adults with obesity or overweight with at least one weight-related condition (such as high blood pressure, type 2 diabetes, or high cholesterol), for use in addition to a reduced calorie diet and increased physical activity…The drug is indicated for chronic weight management in patients with a body mass index (BMI) of 27 kg/m2 or greater who have at least one weight-related ailment or in patients with a BMI of 30 kg/m2 or greater… The largest placebo-controlled trial enrolled adults without diabetes. Individuals who received Wegovy lost an average of 12.4% of their initial body weight compared to individuals who received placebo”
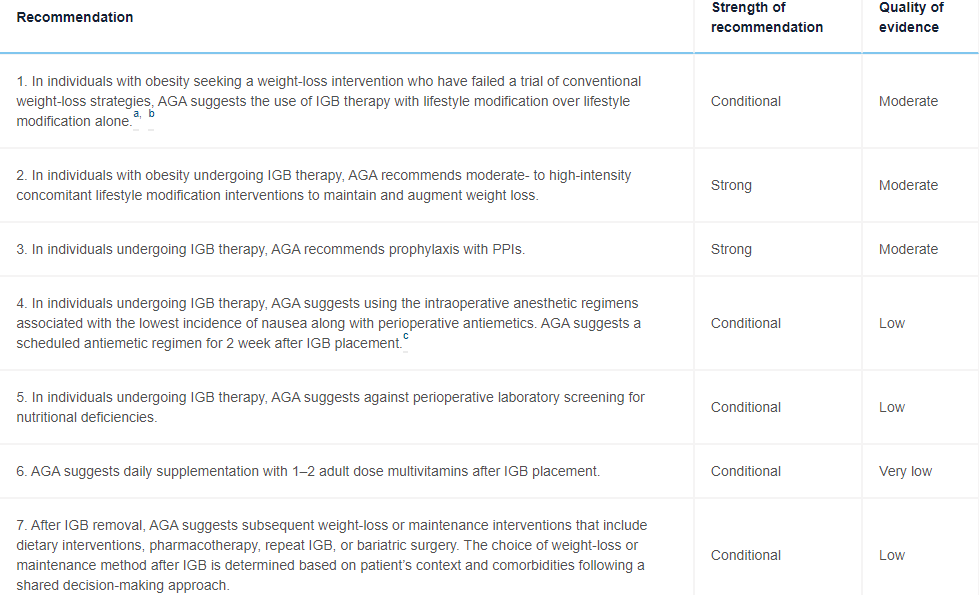
T Muniraj et al. Gastroenterol 2021; 160-1799-1808. Full text: AGA Clinical Practice Guidelines on Intragastric Balloons in the Management of Obesity
Related blog posts:
- Are We On the Verge of Pharmacologic Treatment of Obesity (Again)?
- Semaglutide: Potential or Problematic New Treatment for Fatty Liver Disease/NASH
- Should We Be Excited About a New Medication (Liraglutide) for Obesity?
- In the News: Weight Loss Intragastric Balloons
- FDA Warning for Obesity Intragastric Balloon Devices

