N Uemura et al. Clin Gastroenterol Hepatol 2025; 23: 748-757. Open Access! Vonoprazan as a Long-term Maintenance Treatment for Erosive Esophagitis: VISION, a 5-Year, Randomized, Open-label Study
Background: Potassium-competitive acid blockers, such as vonoprazan, provide more potent gastric acid suppression than proton pump inhibitors. However, long-term safety data are lacking for vonoprazan in patients with healed erosive esophagitis. This study with 208 patients provides long-term data on the use of a vonoprazan.
Methods: Open-label study. Patients with erosive esophagitis (EE) received induction therapy (once daily vonoprazan 20 mg or lansoprazole 30 mg; ≤8 weeks). Those with healed EE received maintenance therapy (once daily vonoprazan 10 mg or lansoprazole 15 mg) for 260 weeks (2:1).
Key findings–Adverse effects:
- No malignant alterations or gastric neuroendocrine tumors (NETs) were observed; there was 1 adenoma in each group
- At week 260, significantly more patients taking vonoprazan vs lansoprazole had parietal cell hyperplasia (97.1% vs 86.5%) and foveolar hyperplasia (14.7% vs 1.9%)
- proportions of patients with ECL cell hyperplasia (4.9% vs 7.7%) and G-cell hyperplasia (85.3% vs 76.9%) were similar
- Median serum gastrin levels were higher with vonoprazan treatment vs lansoprazole (625 pg/mL vs 200 pg/mL)
Key finding –Efficacy:
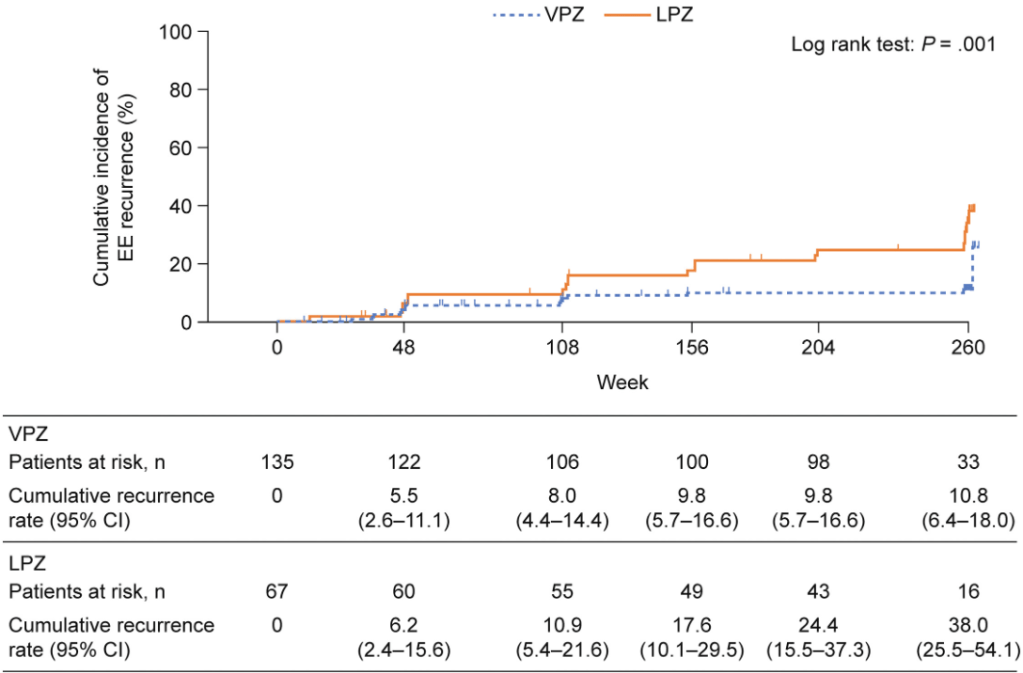
- Overall, the cumulative EE recurrence over 260 weeks was lower in the vonoprazan group (10.8% ) vs the lansoprazole group (38.0%) (P = .001)
Discussion Points:
- “Annual endoscopies and biopsies performed in the VISION study are considered objective approaches for detecting upper gastrointestinal diseases and variable lesions, as well as gastric mucosa morphological changes in areas without endoscopically apparent lesions…Although the proportions of patients with parietal cell protrusion and foveolar hyperplasia were higher in the vonoprazan group than in the lansoprazole group over 5 years, the clinical significance of these findings is unclear.”
- “The safety profiles of vonoprazan and lansoprazole were also comparable, suggesting that long-term use of vonoprazan is as safe as PPIs.”
My take: This study provides some reassurance regarding the risk of using vonoprazan & other potassium-competitive acid blockers. The benefits of controlling erosive esophagitis may outweigh potential safety risks of long-term use. Nevertheless, it will be a while before this class of medications is used extensively in the pediatric age group.
Related blog posts:
- Practice Advice for Potassium-Competitive Acid Blockers (2024)
- Competition for Competitive Acid Blockers
- Vonoprazan Treatment of Heartburn in Randomized Study of Patients with Non-Erosive Reflux Disease
- Is Vonoprazan Better Than Intravenous PPIs for High-Risk Peptic Ulcers?
- Improved Efficacy with Vonoprazan for Severe Esophagitis
- Why Vonoprazan Is More Effective For Erosive Esophagitis Than a Proton Pump Inhibitor
- Understanding FDA Approval of Vonoprazan-Based Therapies for Helicobacter Pylori
- Safety and Efficacy of Potassium Competitive Acid Blockers (3 Studies)
Disclaimer: This blog, gutsandgrowth, assumes no responsibility for any use or operation of any method, product, instruction, concept or idea contained in the material herein or for any injury or damage to persons or property (whether products liability, negligence or otherwise) resulting from such use or operation. These blog posts are for educational purposes only. Specific dosing of medications (along with potential adverse effects) should be confirmed by prescribing physician. Because of rapid advances in the medical sciences, the gutsandgrowth blog cautions that independent verification should be made of diagnosis and drug dosages. The reader is solely responsible for the conduct of any suggested test or procedure. This content is not a substitute for medical advice, diagnosis or treatment provided by a qualified healthcare provider. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a condition.