R Panaccione et al. The Lancet Gastroenterology & Hepatology. 2025; 10: 507 – 519. Open Access! Long-term efficacy and safety of upadacitinib in patients with moderately to severely active ulcerative colitis: an interim analysis of the phase 3 U-ACTIVATE long-term extension study
Methods: U-ACTIVATE is an ongoing, 288-week, phase 3, long-term extension study that enrolled patients (n=369) aged 16–75 years with a confirmed diagnosis of moderately to severely active ulcerative colitis; patients who had a clinical response in the induction studies were eligible to enter the U-ACHIEVE maintenance study. Patients not in clinical remission originally randomly assigned to upadacitinib 15 mg were eligible to escalate to upadacitinib 30 mg, those originally randomly assigned to upadacitinib 30 mg continued on upadacitinib 30 mg, and those originally assigned to placebo were eligible to escalate to upadacitinib 15 mg in a masked way
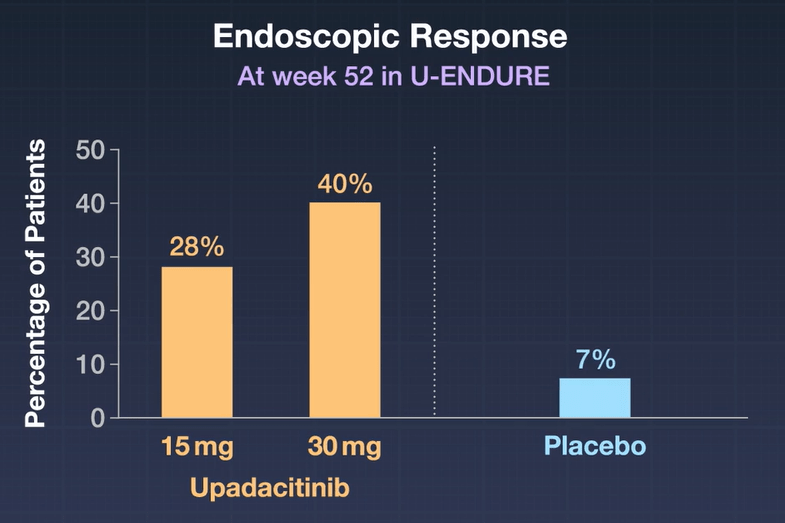
Key findings:
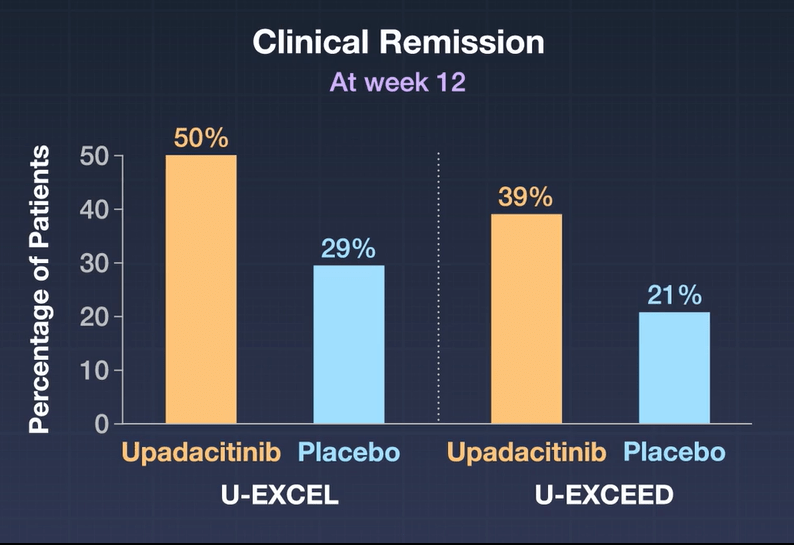
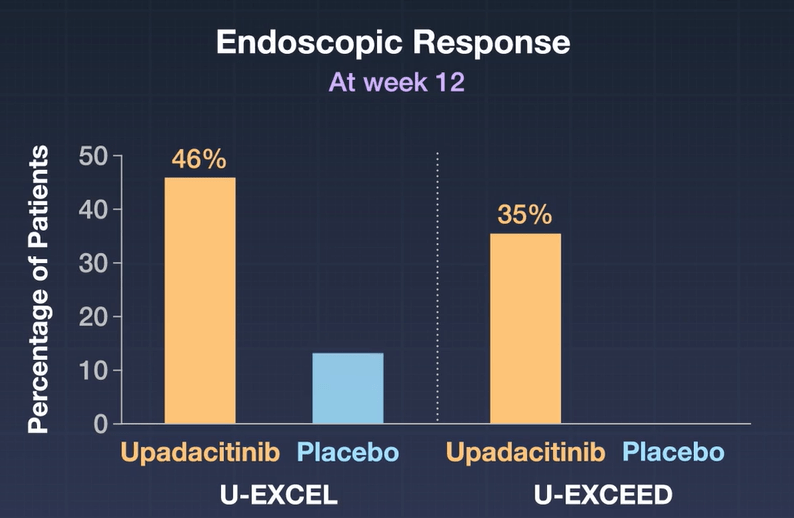
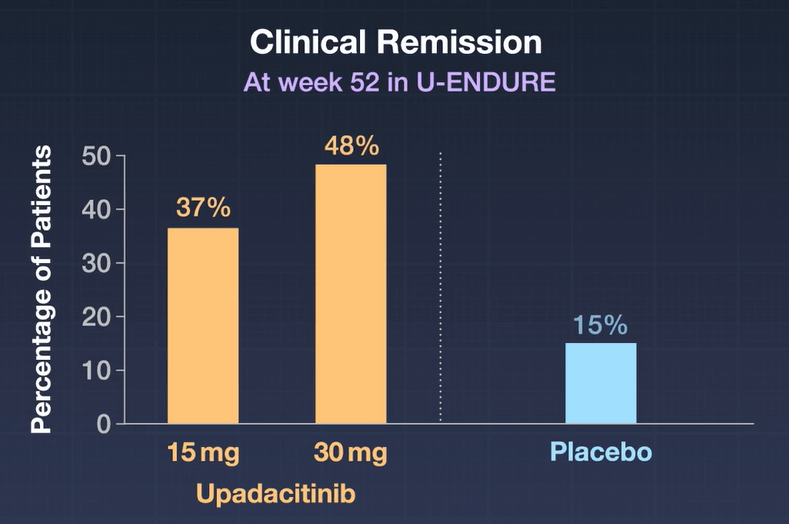
- In the as-observed population, 84 (71%) of 118 patients receiving upadacitinib 15 mg were in clinical remission at week 48, as were 130 (67%) of 193 receiving upadacitinib 30 mg
- By week 96, 69 (76%) of 91 patients receiving upadacitinib 15 mg and 104 (74%) of 141 of those receiving upadacitinib 30 mg were in clinical remission
- The most common adverse events of special interest were hepatic disorder, lymphopenia, creatine phosphokinase elevation, serious infection, neutropenia, and herpes zoster

My take: This study shows a good durable (3 year) response to upadacitinib treatment with both 15 mg and 30 mg dosing.
Related blog posts:
- Comparative Evidence and Positioning Advance Therapies for Inflammatory Bowel Disease
- Upadacitinib vs Ustekinumab for Ulcerative Colitis
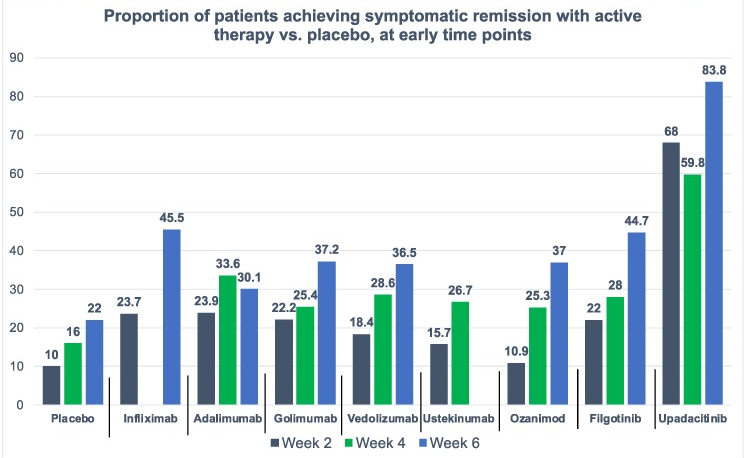
- Upadacitinib Works Quickly and with High Response
- AGA Living Guideline for Moderate-to-Severe Ulcerative Colitis –The Good and The Bad
- Dr. Joel Rosh: Positioning Therapies for Pediatric Ulcerative Colitis
- IBD Brief Updates: Anti-TNF Loss of Response, Upadacitinib for ASUC, Risk Factors for Developing IBD
- IBD Briefs: Upadacitinib in Children, Predicting Crohn’s Disease, and Autoimmune Diseases Associated with IBD
- Upadacitinib Receives FDA Approval to Treat Adults with Ulcerative Colitis