KG Whaley et al. Clin Gastroenterol Hepatol 2023; 21: 1338-1347. Multicenter Cohort Study of Infliximab Pharmacokinetics and Therapy Response in Pediatric Acute Severe Ulcerative Colitis
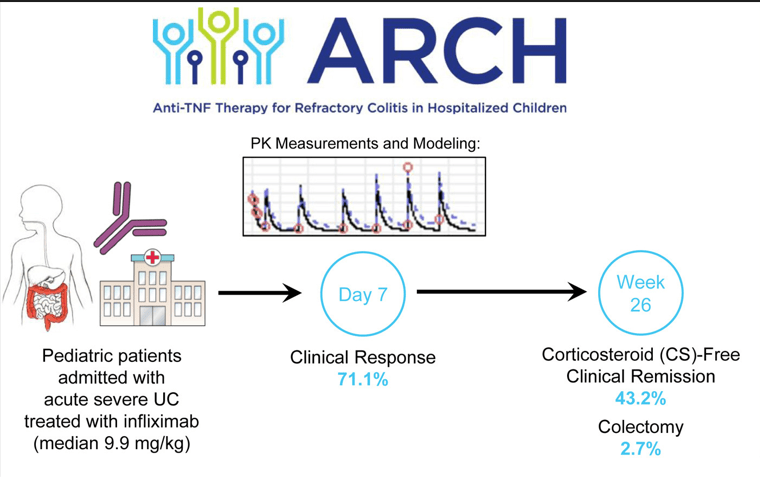
This was a multicenter prospective cohort of hospitalized children initiating IFX for ASUC or IBD-unclassified (n=38).
Key findings:
- Compared to previous publications of pediatric ASUC, there was a low colectomy rate in this cohort of 2.7% at week 26 and 10.8% at 2 years
- Median initial IFX dose was 9.9 mg/kg
- Early rapid clearance was strongly associated with colectomy
- Faster clearance was associated with higher WBC, presence of antibodies to infliximab and lower albumin. Higher platelets were associated with increased volumes of distribution. Concomitant immunomodulator use (26% with methotrexate, 13% thiopurine) “was not a significant covariate for PK parameters”
Discussion points:
- Higher IFX dosing (10 mg/kg) may sufficiently optimize early outcomes in pediatric ASUC. Prior retrospective studies of adult and pediatric ASUC patients have supported lower colectomy rates with intensified induction regimens compared to standard induction regimens
- The availability of vedolizumab may also have contributed to a lower colectomy rate
- WBCs, “specifically neutrophils, may participate in the elimination of IFX”
- Limitations: observational study, lack of dose standardization, lack of endoscopic outcomes
My take: Especially in pediatric patients, there is ample data to support using 10 mg/kg dosing for infliximab in patients with more severe inflammatory bowel disease, both ulcerative colitis and Crohn’s disease.
Related blog posts:
- Another Study Justifying Higher Infliximab Dosing in Pediatrics
- “For Hospitalized Patients With ASUC, 5-ASA Adds No Value to Steroids”
- Treatments for “Bad” Inflammatory Bowel Disease (Part 2) & Reassuring Data on Tofacitinib
- @MondayNightIBD and Acute Severe Ulcerative Colitis Algorithm
- A Definite Maybe: Antibiotics for Acute Severe Colitis
- AGA Guidelines: Moderate to Severe Ulcerative Colitis
- Disease extent and need for higher infliximab dosing
- Higher Stool Infliximab Correlates with Poor Response in Severe Ulcerative Colitis
- “Denials, Dilly-dallying and Despair”
- Kids Are Different: Therapeutic Drug Monitoring
- Expert Guidance on Inflammatory Bowel Disease (Part 3)
- Modeling Trough Levels to Predict Optimal Infliximab Dosing
- Improving Outcomes with Proactive Therapeutic Drug Monitoring (JAMA 2021 study)