Home | About Jay Hochman -Pediatric Gastroenterology Blog | Archives
July 6, 2023 7:00 am
MD Kappelman et al. Gastroenterology. 2023: 165: 149-161. Open Access! Comparative Effectiveness of Anti-TNF in Combination With Low-Dose Methotrexate vs Anti-TNF Monotherapy in Pediatric Crohn’s Disease: A Pragmatic Randomized Trial
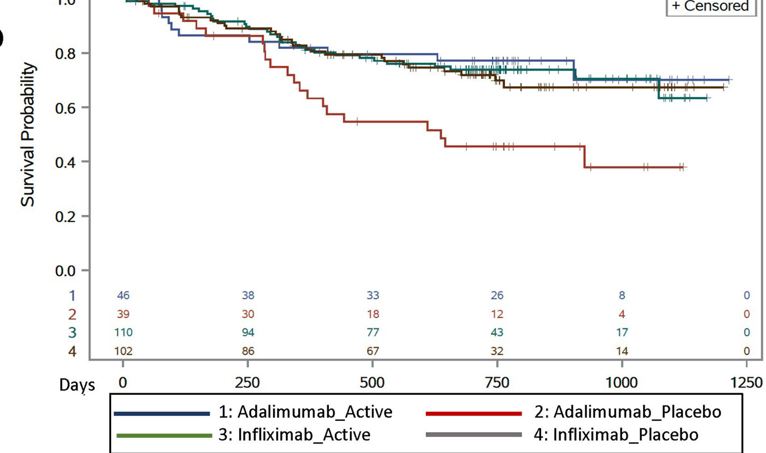
This study enrolled 297 children with Crohn’s disease starting anti-TNF therapy. Patients initiating infliximab or adalimumab were randomized in 1:1 allocation to methotrexate or placebo and followed for 12–36 months.
Methotrexate dosing: For those in the active arm, oral methotrexate was administered with a weekly dose of 15 mg for children ≥40 kg, 12.5 mg for children 30 to <40 kg, and 10 mg for children 20 to <30 kg. All participants received pretreatment with ondansetron 4 mg (or placebo) to prevent nausea and folic acid (1 mg/d).
Key findings:
My thoughts on this study:
My take: Given the increased difficulty monitoring the kids on adalimumab, they are probably better off on dual therapy. My suspicion, though, is that if they had optimized levels, the benefit of dual therapy is probably small and would mirror the findings with IFX.

Posted by gutsandgrowth
Categories: Pediatric Gastroenterology Intestinal Disorder
Tags: combination therapy, Crohn's disease, dual therapy, inflammatory bowel disease, Methotrexate
Mobile Site | Full Site
Get a free blog at WordPress.com Theme: WordPress Mobile Edition by Alex King.