B Bokemeyer et al. Inflamm Bowel Dis 2024; 30: 746-756.
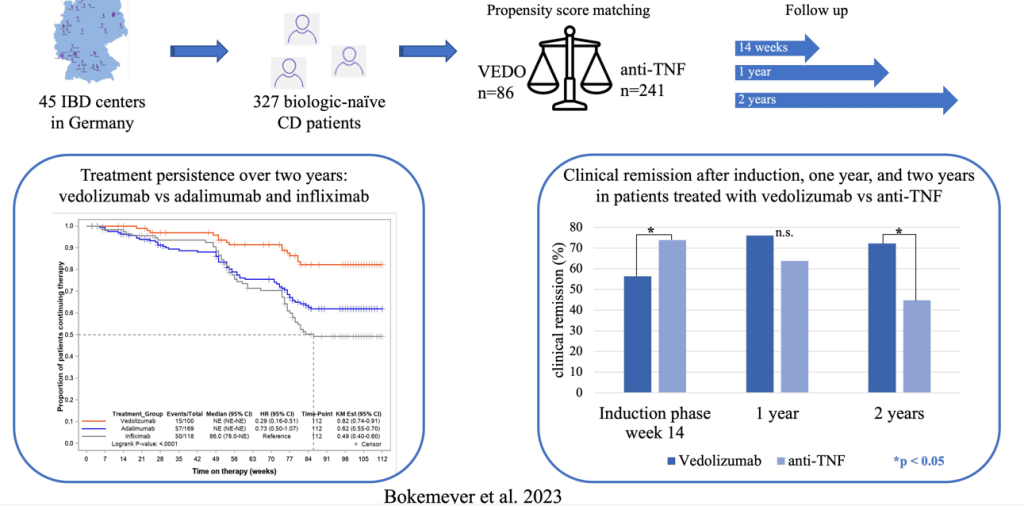
Methods: 3277 adult biologically-unexposed CD patients starting therapy with VEDO or anti-TNF were consecutively enrolled in 45 IBD centers across Germany (2017-202). This was a non-randomized, observational study with prospectively collected data.
Findings:
- Anti-TNF agents had higher induction clinical remission rates compared to vedolizumab: 73.9% 56.3% vs, P < .05
- Vedolizumab (VEDO) had higher long-term clinical remission rates: clinical remission after 2 years was significantly better for VEDO compared with anti-TNF, 74.2% vs 44.7%; P < .05. This was associated with a much better treatment persistent rate. The switch rate for VEDO was 17% compared with 44% for anti-TNF agents.
- Among week 14 responders, VEDO 2-year clinical remission rates were 88.6% compared to 45.8% (P < .00001) for anti-TNF agents
The discussion describes the strengths and limitations of this study. As it is not a randomized control trial, there can still be selection bias and confounding even with propensity scoring that was done in this study. The authors note that in a prior analysis of RCTs comparing infliximab to vedolizumab in CD patients, that infliximab had higher efficacy for induction and maintenance, though the clinical remission rates were only modestly improved at 1 year. (L Peyrin-Biroulet et al. BMC Gastroenterol 2022; 22: 291).
Recent expert guidance (2024) has favored infliximab and risankizumab over other advance therapies in CD patients who have not had previous biologic therapies (see: Comparative Evidence and Positioning Advance Therapies for Inflammatory Bowel Disease).
My take: This study shows that vedolizumab is a good advanced therapy for patients with Crohn’s disease without prior therapy. Among those with a clinical response at 14 weeks, the treatment durability was particularly impressive in this cohort.
It would be great to see an RCT in children with CD comparing IFX to VEDO. Treatment persistence is even more important in younger patients.
Related blog posts:
- Comparative Evidence and Positioning Advance Therapies for Inflammatory Bowel Disease
- SC Infliximab versus Vedolizumab for Crohn’s Disease and for Ulcerative Colitis
- ENTERPRISE Study: Vedolizumab for Perianal Fistulizing Crohn’s Disease
- Ustekinumab Over Vedolizumab as 2nd Line Agent for Crohn’s Disease
- Dr. Joel Rosh: Positioning Therapies for Pediatric Ulcerative Colitis (2024)
- Impressive Results for Risankizumab in Refractory Crohn’s Disease (2024)
- Vedolizumab vs Adalimumab: Histology Outcomes from Varsity Trial, Vedolizumab More Effective Than Adalimumab for Ulcerative Colitis
- IBD Updates: Preventing Inflammatory Bowel Disease with a Healthy Diet and Medication Safety Pyramid
- ARCH Study: Higher Doses of Infliximab in Acute Severe Ulcerative Colitis
- Landmark Study: Oral Biologic for Crohn’s –Upadacitinib
- CCFA 2023 (Atlanta) -Part 1
- CCFA 2023 (Atlanta) Part 4
