E Ricciuto et al. Aliment pharmacol ther 2024; 59: 1236-1247. Oral vancomycin is associated with improved inflammatory bowel disease clinical outcomes in primary sclerosing cholangitis-associated inflammatory bowel disease (PSC-IBD): A matched analysis from the Paediatric PSC Consortium
This was a retrospective study from 54 centers with 113 PSC-IBD pediatric patients receiving vancomycin (median age 12.7 years, 63% male). The matched cohort included 70 vancomycin-treated and 210 untreated patients. Clinical remission was defined as physician global assessment (PGA) of zero. It is noted that the Pediatric PSC consortium included 1362 patients at the time of this study; only 11% (n=113) were treated with vancomycin for at least 3 months. The median dose of vancomycin was 17 mg/kg/day and median duration was 2.5 years.
Key findings:
- Vancomycin was associated with greater odds of IBD clinical remission (odds ratio [OR] 3.52, 95% CI 1.97-6.31; adjusted OR [aOR] 5.24, 95% CI 2.68-10.22).
- Vancomycin was associated with increased odds of endoscopic remission (aOR 2.76, 95% CI 1.002-7.62; N = 101 with data), and with lower CRP (p = 0.03) and higher hemoglobin and albumin (both p < 0.01).
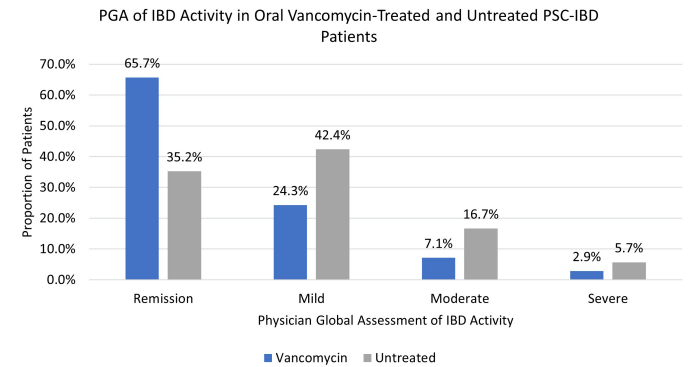
- At baseline, prior to vancomycin, 34% (30/88) were in clinical remission; this increased to 60% (52/86) after 6 months of treatment. After ~ 1 year, 71% (55/78) of children treated with vancomycin were in remission, compared with 35% who had not receive the antibiotics.
- Ursodeoxycholic acid use: 53% for vancomycin-treated and 82% of control group (P<0.001). Other cotherapies were similar including infliximab (36% vs. 27%) and vedolizumab (13% vs 7%)
- Only 28 vancomycin-treated patients had baseline and f/u colonoscopy data available. 46% of this subgroup had endoscopic remission compared to 26% of matched untreated controls.
In the discussion, the authors acknowledge the limitations of a retrospective observational study. RCTs are quite difficult with rare disorders, especially in children. In addition, the exact mechanisms for vancomycin efficacy remain unclear -possibly microbial changes or its effects on bile acids. They note that many patients treated with vancomycin had mild clinical activity at baseline. Though, even this population may benefit with resolution of clinical inflammation which could reduce the risk of colorectal cancer.
My take: In patients with PSC-IBD, the use of vancomycin for IBD should be a consideration especially in those who have not responded adequately to other treatments.
Related blog posts:
- PSC in IBD
- Recurrent PSC in Children After Liver Transplantation
- Aspen Webinar 2021 Part 5 -Autoimmune Liver Disease & PSC 2021. This lecture highlights studies show lack of efficacy with vancomycin, ursodeoxycholic acid and vedolizumab in altering the liver disease. Also, there is potential utility of MMP-7 for distinguishing between PSC and AIH
- Liver Problems with Inflammatory Bowel Disease
- ctive Colitis More Likely in Children with PSC-IBD
- Big Study of PSC in Children
- PSC -Natural History Study (pediatric)
- Should We Care About Subclinical Primary Sclerosing Cholangitis with Inflammatory Bowel Disease?
- Primary Sclerosing Cholangitis (PSC) –Natural History Study
