RS Boneh et al. Clin Gastroenterol Hepatol 2025; 23: 2001-2011. Open Access! Modified Crohn’s Disease Exclusion Diet Maintains Remission in Pediatric Crohn’s Disease: Randomized Controlled Trial
In this “DIETOMICS” study with 56 children with mild-to-severe Crohn’s disease, after a 2 week exclusive enteral nutrition (EEN) diet, 30 patients were randomized to CDED and 26 to EEN.
Diet intervention: The CDED group followed 3 diet phases over 24 weeks: phase 1 (weeks 3–8) supplemented with 50% PEN; phase 2 (weeks 9–14) with 25% PEN, as described previously16; and phase 3 (weeks 15–24) with gradual introduction of more foods, including 1 and 2 free meals per week from weeks 15 and 18, respectively.17 Patients in EEN group received 8 weeks of EEN followed by gradual introduction of free diet with 25% PEN up to week 24.
Key findings:
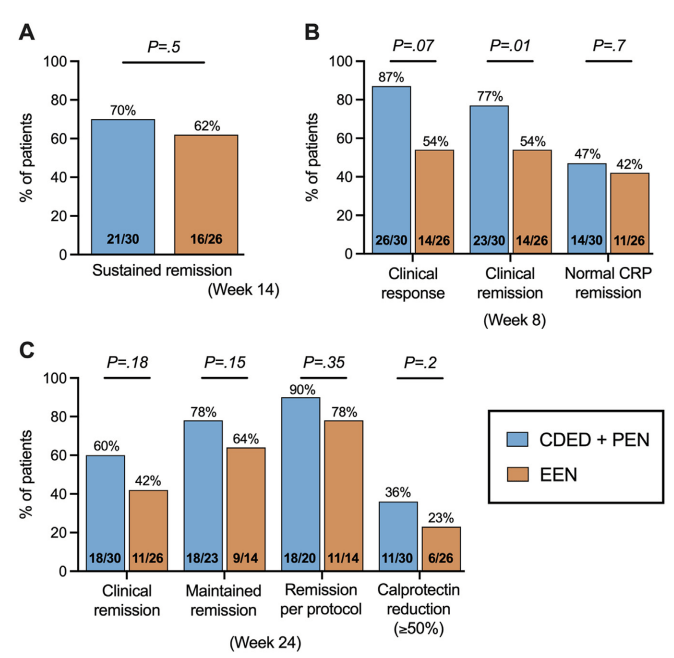
This study with a relatively small number of enrolled patients had a lot of variables in dietary parameters. “An additional potential confounder in this study is the use of IMM therapy. Although both groups were recommended to initiate IMM therapy from weeks 4 to 5 to maintain remission, several CDED patients opted for monotherapy with CDED and preferred to delay medication initiation. Interestingly, 90% of patients on CDED without IMM therapy were in remission at week 14 and 100% were in remission at week 2” (possibly impacting decision not to use IMM).
My take: This study adds another piece of information to the puzzle on dietary therapy for Crohn’s disease. The authors note the following: “while CDED shows promise as a standalone therapy in some cases, in more severe cases it may be more appropriately as an adjuvant to top-down treatment with early anti-TNF.4 Recent research and guidelines advocate for a top-down approach (anti-TNF ± nutrition) for more severe disease, emphasizing the integration of anti-TNF therapy with nutrition.8,29 This approach is crucial during critical growth stages, as the conventional step-up method may lead to ineffective use of IMM with prolonged steroid exposure and growth issues.12“
Related blog posts:
- “Tasty & Healthy” Whole Food Diet For Crohn’s Disease
- AGA Guidance: Nutritional Therapies for Inflammatory Bowel Disease
- The Quality of Evidence for Dietary Treatments in Inflammatory Bowel Disease
- CDED + PEN: An Alternative Diet to Exclusive Enteral Nutrition?
- Dr. Maria Oliva-Hemker: Positioning Therapies for Pediatric Crohn’s Disease
- More Evidence That A Proinflammatory Diet May Increase the Risk of Crohn’s Disease
- Pushing the Boundaries on Dietary Therapy for Crohn’s Disease (CD-TREAT)
- Predicting Enteral Nutrition Therapy Response in Patients with IBD
- Dietary Therapy for Adults with Crohn’s Disease
- Trial by Diet Approach for Crohn’s Disease in Children
- Dietary Therapy for Inflammatory Bowel Disease –Useful Update
- Mediterranean Diet vs Specific Carbohydrate Diet for Crohn’s Disease
- Mediterranean Diet’s Impact on Crohn’s Disease Outcomes
Disclaimer: This blog, gutsandgrowth, assumes no responsibility for any use or operation of any method, product, instruction, concept or idea contained in the material herein or for any injury or damage to persons or property (whether products liability, negligence or otherwise) resulting from such use or operation. These blog posts are for educational purposes only. Specific dosing of medications (along with potential adverse effects) should be confirmed by prescribing physician. Because of rapid advances in the medical sciences, the gutsandgrowth blog cautions that independent verification should be made of diagnosis and drug dosages. The reader is solely responsible for the conduct of any suggested test or procedure. This content is not a substitute for medical advice, diagnosis or treatment provided by a qualified healthcare provider. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a condition
