- ES Dellon et al. Clin Gastroenterol Hepatol 2025; 23: 2155-2166. Open Access PDF! Long-term Safety and Efficacy of Budesonide Oral Suspension for Eosinophilic Esophagitis: A 4-Year, Phase 3, Open-Label Study
- L Biedermann et al. Clin Gastroenterol Hepatol 2025; 23: 2144-2154. Open Access! Efficacy and Safety of Budesonide Orodispersible Tablets for Eosinophilic Esophagitis up to 3 Years: An Open-Label Extension Study
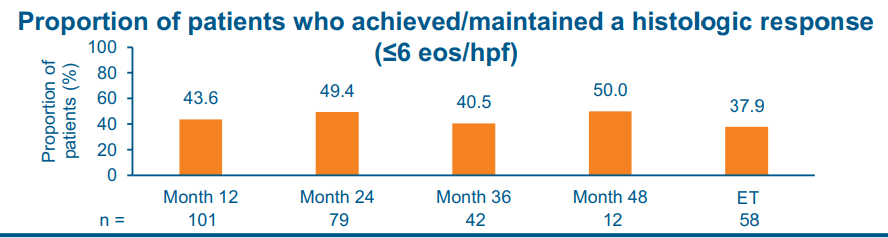
The study by Dellon et al was a 4-year, phase 3, open-label study in patients with EoE who completed up to 52 weeks of BOS therapy (Budesonide oral suspension 2 mg 2/day) in 2 preceding phase 3 studies.
Key findings:
- At month 48 of treatment, 50.0% and 58.3% of patients achieved/maintained a histologic response (< or =6 and <15 eosinophils per high-power field, respectively)
Safety:
- Treatment-emergent adverse events (TEAEs) occurred in 76.3% of patients; most were mild/moderate in severity and unrelated to study drug.
- The most frequently reported BOS-related TEAEs included abnormal adrenocorticotropic hormone stimulation test results (8.4%, 11/131; number of events [m] [ 12) and adrenal insufficiency (2.3%, 3/131; m [ 3). Esophageal candidiasis occurred in 3.1% of patients (4/131)
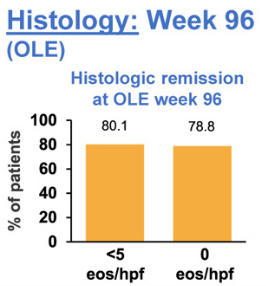
The study by Biedermann et al explored the use of an orodipsersible tablet for EoE up to 3 years in patients who achieved remission during a 12-week induction. This tablet is not available in the U.S.
Key findings:
- At week 96, 80.1% of patients were in histologic remission, defined as peak eosinophils per high-power field of <5, at week 96 vs 91.8% at open label extension baseline
- No new safety concerns were observed across 96 weeks of treatment. Suspected symptomatic candidiasis occurred at similar rates to prior BOT studies and was predominantly mild and resolved with treatment
My take: The pharmaceutical budesonide suspension, Eohilia, is labelled by the FDA for use as a 12 week treatment course. Since EoE is a chronic disease, 12 weeks is insufficient. These long-term studies provide data that may address this shortcoming.
Related blog posts:
- Budesonide FDA-Approved for Eosinophilic Esophagitis
- Budesonide Tablet vs Off-Label Corticosteroids in Eosinophilic Esophagitis
- Briefly noted: Aerodigestive Medicine and Budesonide for Eosinophilic Esophagitis
- Eosinophilic Esophagitis -Increasing Incidence and Emergence of Biologic Treatments
- NASPGHAN YouTube Video for Eosinophilic Esophagitis
- Long-Term Treatment of Eosinophilic Esophagitis with Budesonide
- Budesonide for Maintaining EoE Remission
- Head-to-Head: Budesonide vs Fluticasone for Eosinophilic Esophagitis
- Orodispensable Budesonide Tablets for Eosinophilic Esophagitis
- Surprising Findings in Prospective Budesonide-Eosinophilic Esophagitis Study
- Practical Guide to Dietary Therapy for Eosinophilic Esophagitis
- When to Use Dupilumab for Eosinophilic Esophagitis: Multispecialty Guidelines
- Managing Adrenal Insufficiency in Eosinophilic Esophagitis