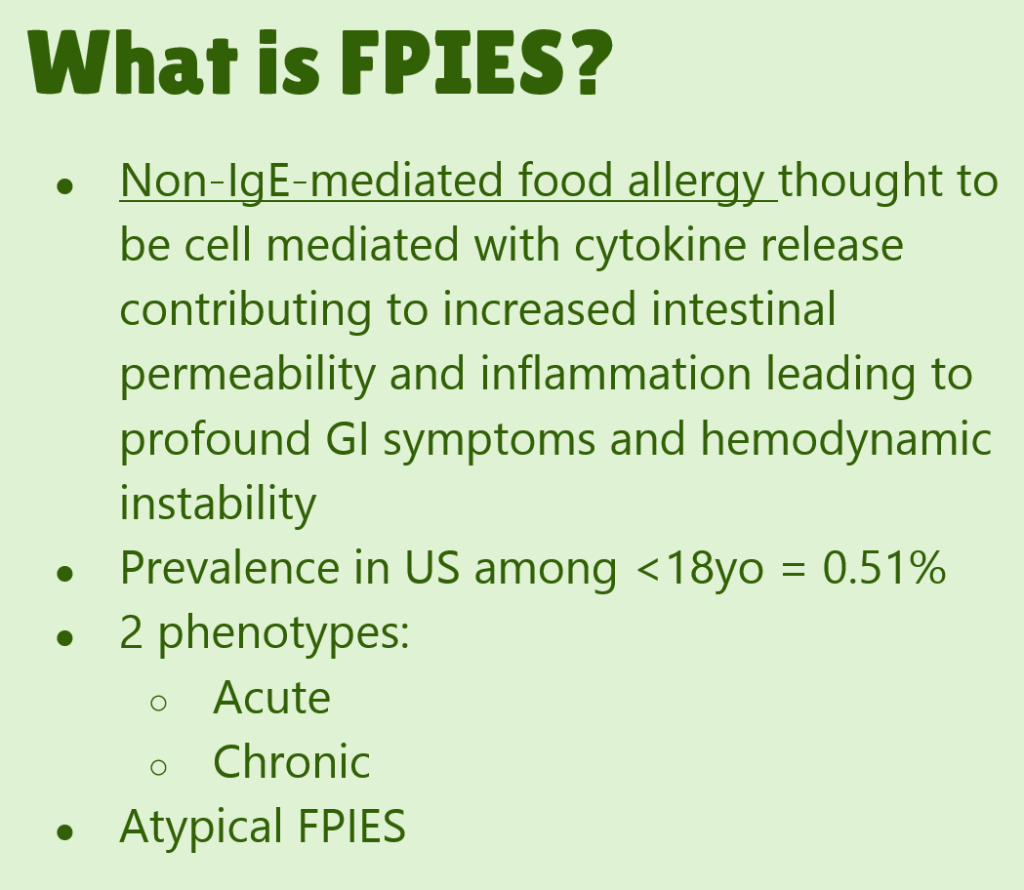
Recently, international consensus guidelines (A Nowak-Wegrzyn et al. J Allergy Clin Immunol 2017; 139: 1111-26) for the diagnosis and management of food protein-induced enterocolitis (FPIES) have been published.
The report starts with a review of epidemiology and diagnosis. Table 1 outlines features:
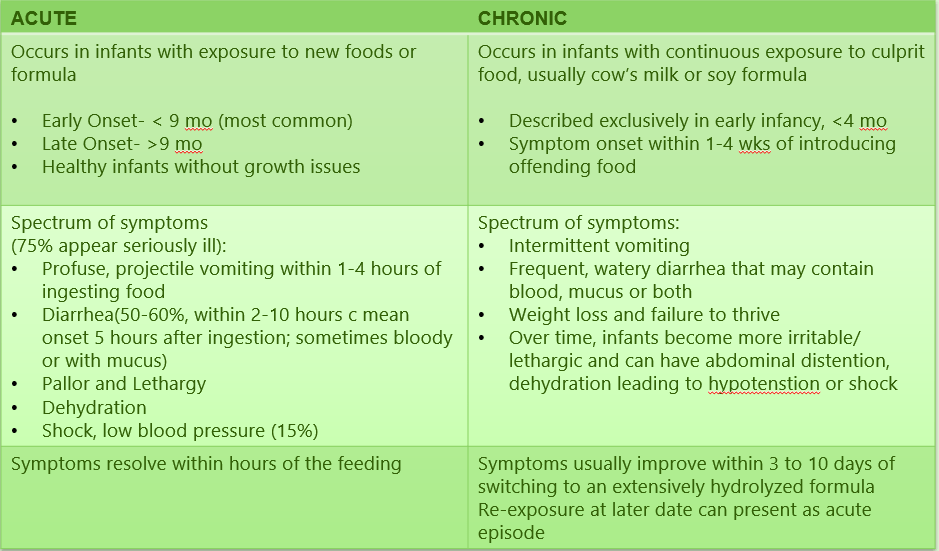
- early vs. late: <9 months or >9 months
- severity: mild-to-moderate =repetitive emesis with or without diarrhea, mild lethargy, severe =repetitive projectile emesis, pallor, lethargy, dehydration, hypotension
- timing: acute vs chronic. Acute occurs with intermittent exposures with emesis 1-4 h following exposure. Chronic occurs with repetitive food exposures (eg. formula in young infants)
- IgE positivity: classical FPIES is IgE negative. Atypical FPIES is IgE positive
Some recommendations:
- #4. “Consider specific IgE testing of children with FPIES to their trigger food because comorbid IgE-mediated sensitization to triggers, such as CM [cow’s milk], can infer a greater chance of persistent disease.”
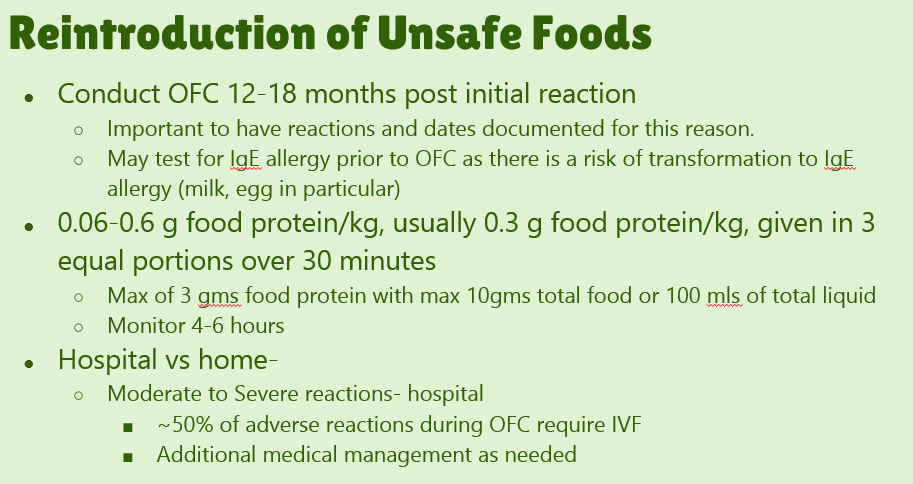
- #8. Conduct food challenges “in patients with suspected FPIES in medically supervised settings in which access to rapid fluid resuscitation is available and prolonged observation can be provided, if necessary.”
- #14. Do not routinely obtain endoscopic evaluation as part of the evaluation of FPIES.
- #17. Acute FPIES should be considered a medical emergency. “Approximately 15% of patients can have hypovolemic shock.”
- #19. Consider ondansetron treatment as an adjunct (if >6 months of age)
- #21. Do not recommend routine maternal dietary elimination of offending triggers while breast-feeding if the infant is asymptomatic.
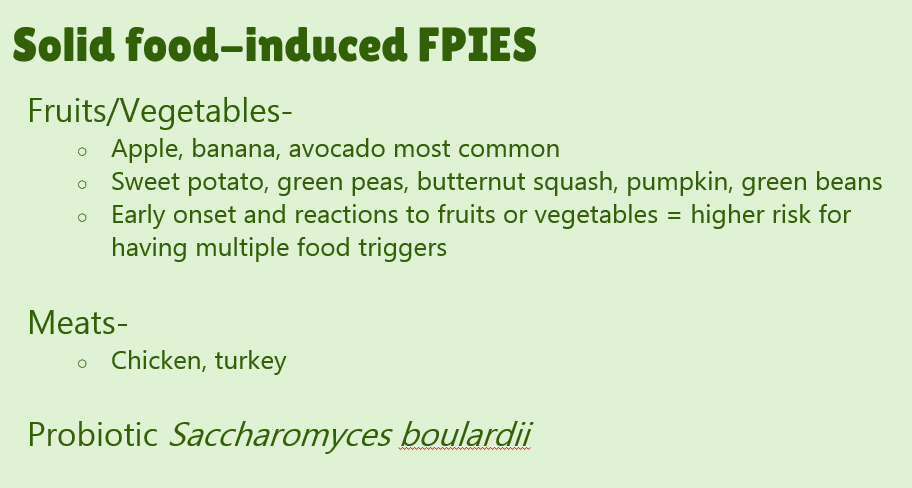
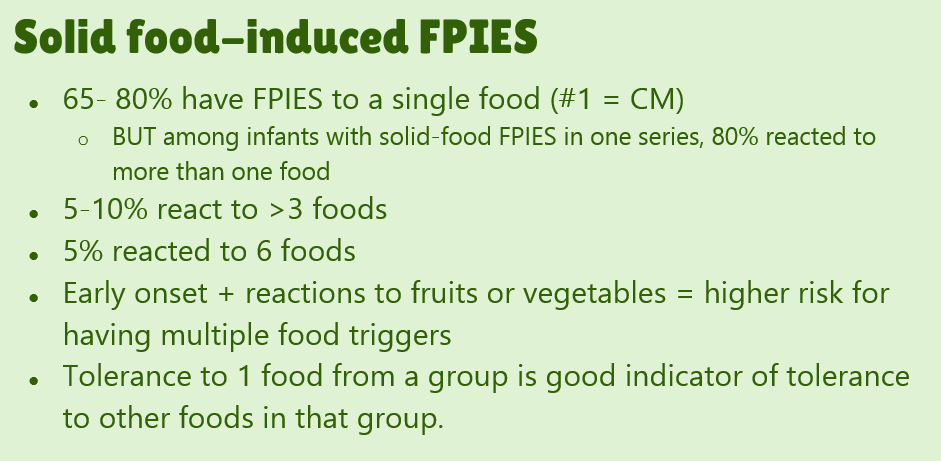
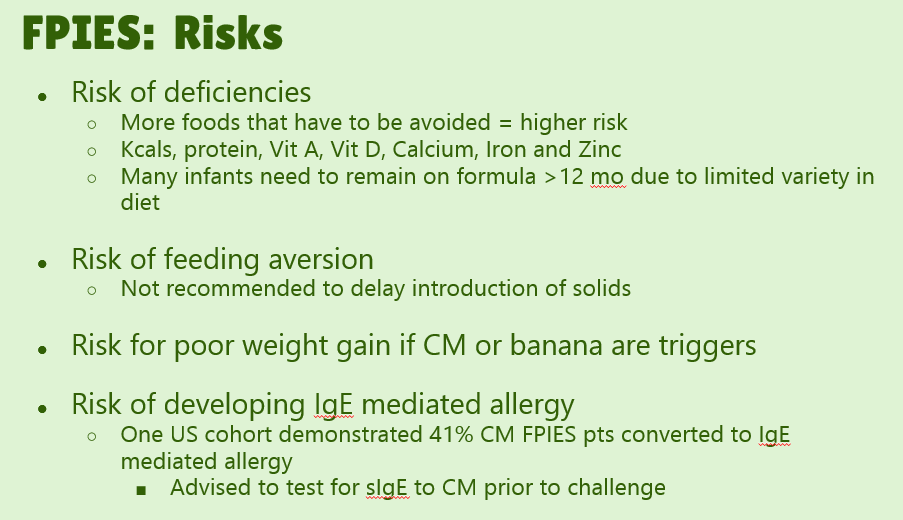
- #23. FPIES can occur to multiple foods. “The majority of children (65% to 80%) have FPIES to a single food, most commonly CM.” In one study, 5% to 10% of children reacted to more than 3 foods.
- #26. Use hypoallergenic formula in infants who can no longer breast-feed and are given a diagnosis of FPIES caused by CM. Most will tolerate extensively hydrolyzed formulas; some may require an amino acid based formula
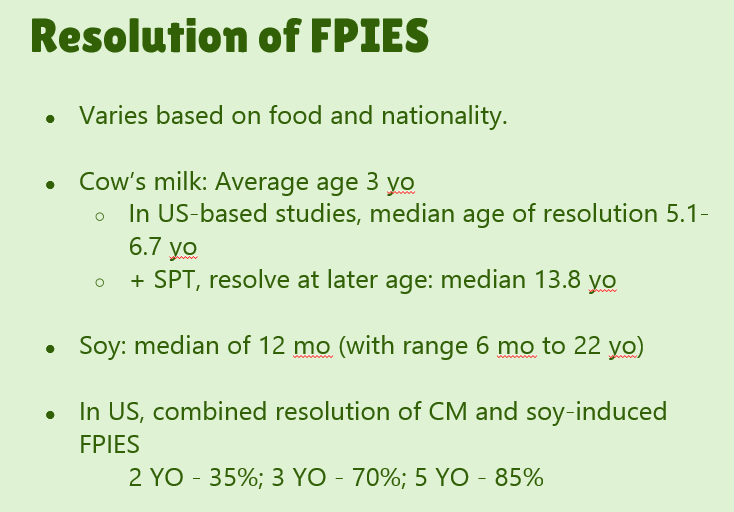
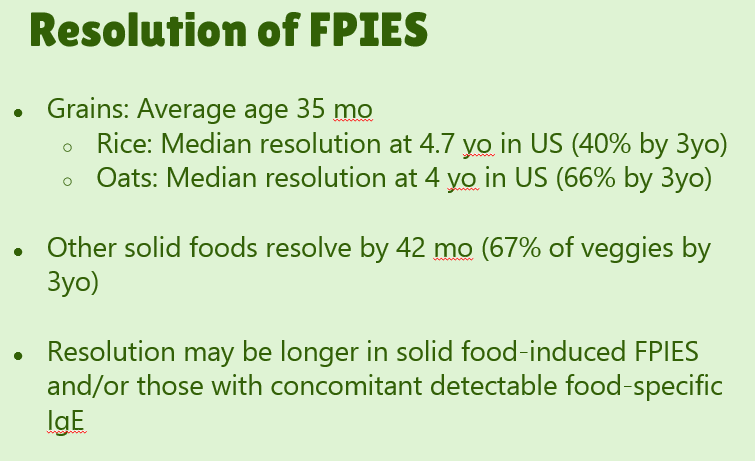
- #29. Reviews natural history. “The age of CM tolerance appears to be around 3 years” but there has been variability in reports. For FPIES due to grains, average age of tolerance is 35 months and other solid foods is 42 months. The average age for soy is 12 months (later in some studies), for rice 4.7 years and 4.0 years for oats. For CM-FPIES with positive SPT response, a much protracted course has been reported, with older age of tolerance (~13.8 years)
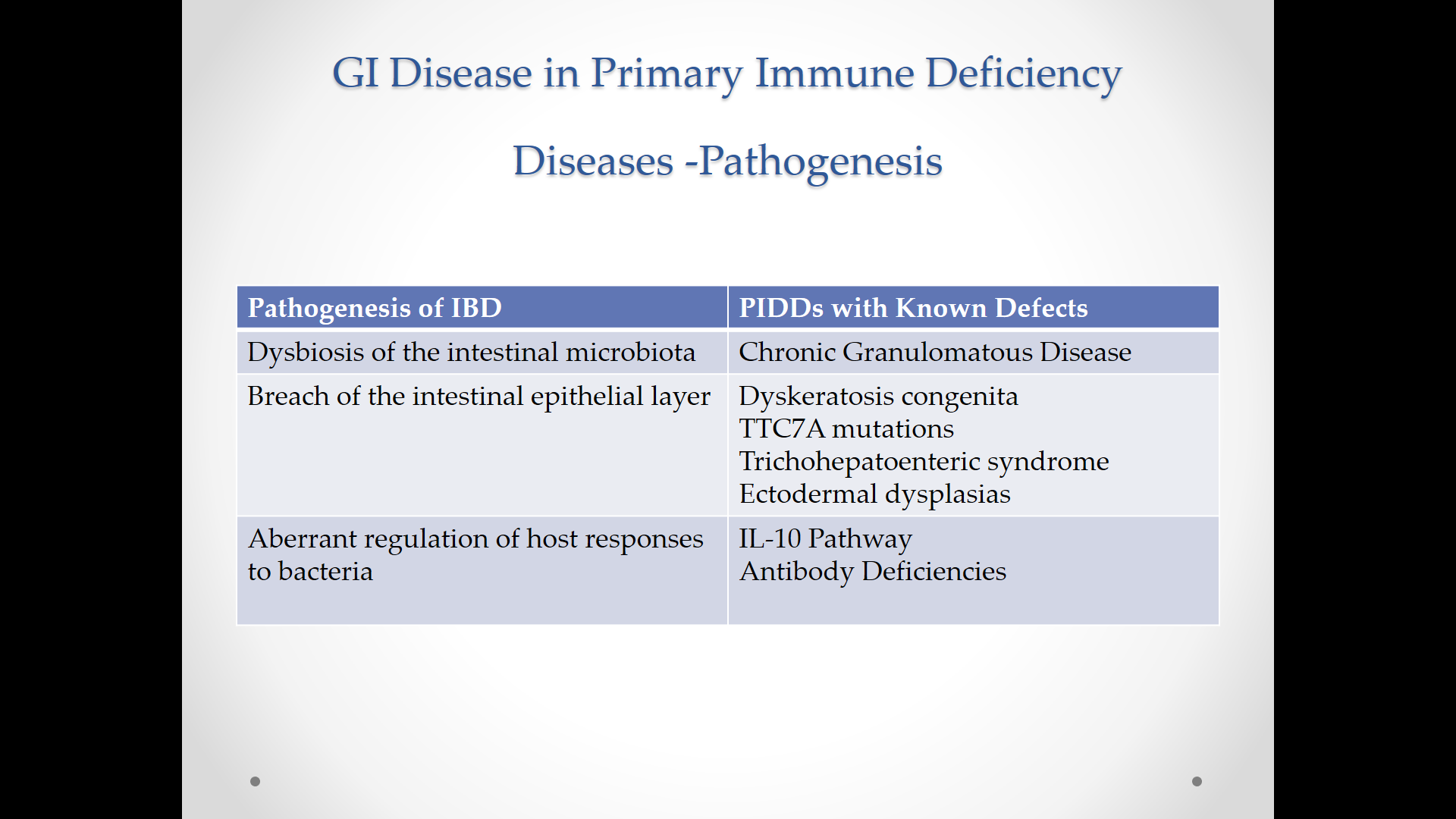
Table III lists a differential diagnosis for FPIES and distinguishing features. This list includes gastroenteritis, necrotizing enterocolitis, anaphylaxis, food aversions, inborn errors of metabolism, cyclic vomiting/neurologic disorders, gastroesophageal reflux, Hirschsprung’s enterocolitis, eosinophilic gastroenteritis, celiac disease, immune enteropathies/IBD, intestinal obstruction, and primary immune deficiencies. Not listed on this table, but worth a mention, would be medical child abuse (aka Munchausen syndrome by proxy).
With regard to inborn errors of metabolism, these include urea cycle defects, hereditary fructose intolerance, hyperammonemic syndromes, Beta-oxidation defects, proprionic/methylmalonic academia, mitochondrial defects and others. Typically, features could include developmental delay, neurologic manifestations, organomegaly, and in some reaction to fruits.
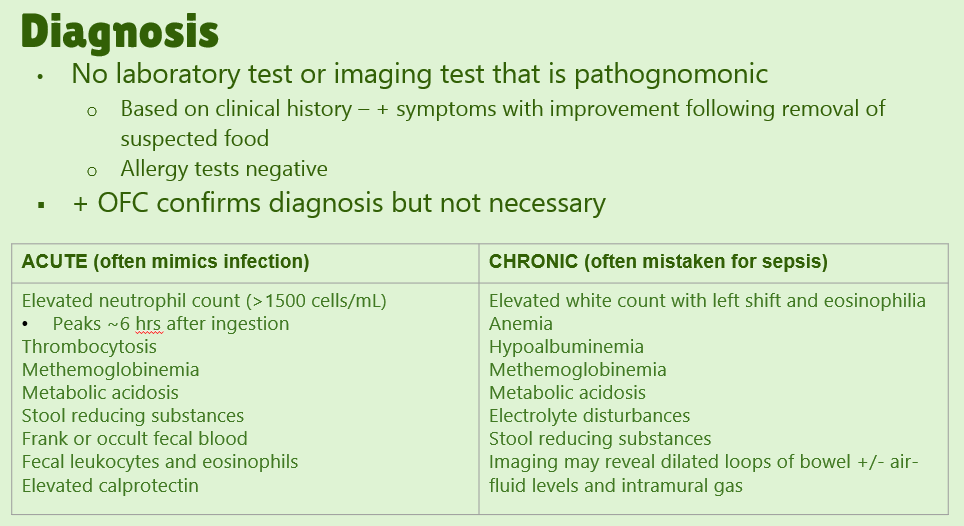
Table IV specifies diagnostic criteria with the major criteria for acute FPIES: vomiting 1- to 4-h period after ingestion of the suspect food and absence of classic IgE-mediated allergic skin or respiratory symptoms. Minor criteria include extreme lethargy, pallor, need for emergency room evaluation/IV fluids, and diarrhea in 24 h (usually 5-10 h).
Table VI details management of FPIES. With moderate bouts, IV fluids with 20 mL/kg normal saline is recommended. For severe episodes, “consider administering intravenous methylprednisolone, 1 mg/kg; maximum 60-80 mg/dose” in addition to fluid resuscitation.
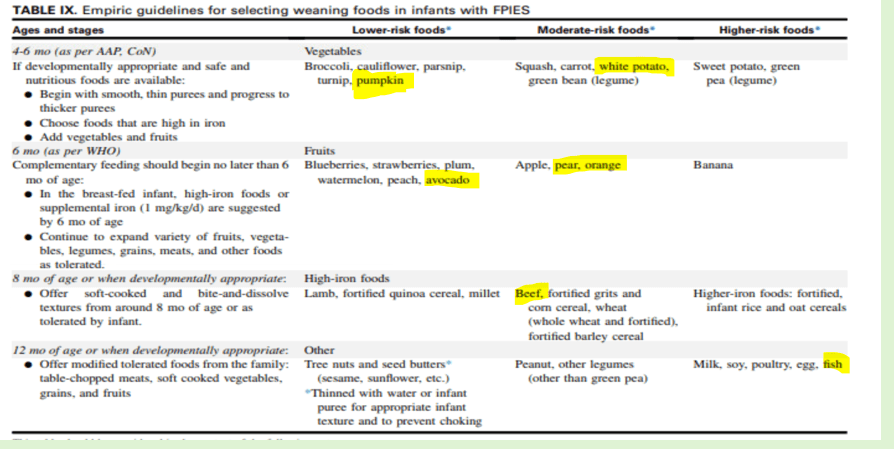
Table IX provides empiric guidelines for selecting weaning foods in infants with FPIES. The recommendations need to be considered based on whether the infant has shown tolerance for a number of foods, which can indicate the acceptability of a more liberal approach. Age-specific guidance:
4-6 months:
- Begin with smooth, thin purees and progress to thicker purees
- Lower-risk foods: vegetables, broccoli, cauliflower, parsnip, turnip, pumpkin
- Moderate-risk: squash, carrot, white potato, green bean
- Higher-risk: sweet potato, green peas
6-8 months:
- Continue to expand vegetables and fruits; in breast-fed, high-iron foods and/or supplemental iron are needed (1 mg/kg/day)
- Lower-risk: fruits, blueberries, strawberries, plum, watermelon, peach, avocado
- Moderate-risk: apple, pear, orange
- Higher-risk: banana
8-12 months:
- Offer soft-cooked and bite-and-dissolve textures
- Lower-risk: high iron foods, lamb, fortified quinoa, millet
- Moderate-risk: beef, fortified grits, corn cereal, wheat, barley
- Higher-risk: fortified infant rice and oat cereals
12 months:
- Offer tolerated table foods: chopped meats, soft vegetables, grains
- Lower-risk: tree nuts
- Moderate-risk: peanut, other legumes (besides green pea)
- Higher-risk: milk, soy, poultry, egg, fish
Overall, with regard to food introduction: While children with FPIES have increased reactions to other foods, “current early feeding guidelines do not recommend delay in introducing complementary foods past 6 months of life because of FPIES. A practical ordering for introducing solids at about 6 months of age at home could start with fruits and vegetables.” For infants with history of severe reactions, “supervised (eg. in-office) introduction can be considered…and prevent unnecessary avoidance.” As with new foods in the home setting, starting with small amounts is recommended and then gradual build up in serving size.
Related blog post: SEED Journal Club: FPIES
Disclaimer: These blog posts are for educational purposes only. Specific dosing of medications (along with potential adverse effects) should be confirmed by prescribing physician. This content is not a substitute for medical advice, diagnosis or treatment provided by a qualified healthcare provider. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a condition.