SN Hong et al. AP&T 2024; 0:1–10. doi.org/10.1111/apt.18354. Subcutaneous Infliximab Concentration Thresholds for Mucosal and Transmural Healing in Patients With Crohn’s Disease
Background: The exposure–response relationship for the intravenous (IV) formulation of infliximab is well established, with multiple studies demonstrating that higher trough concentrations (C-trough) are associated with improved patient outcomes…However, the 2-week cycle of subcutaneous administration showed many-fold higher C-trough than the 8-week cycle of IV-IFX. Direct comparison of C-trough between SC- and IV-IFX is not appropriate because of different bioavailability and concentration–time profile. It is also not appropriate to apply the C-trough thresholds that predict achieving the therapeutic targets for IV.
This was a cross-sectional retrospective study with 124 patients with Crohn’s disease (CD) who had received SC-IFX maintenance therapy for ≥6 months. SC-IFX C-trough was measured immediately before SC-IFX injection. Key findings:
- Mucosal healing (MH) was noted in 77.9% (74/95) and transmural healing (TH) in 36.3% (37/102).
- SC-IFX C-trough was significantly higher in patients with MH (24.1 vs.16.9 μg/mL; p=0.001) and TH (26.0 vs. 20.5 μg/mL; p=0.007) than in those without.
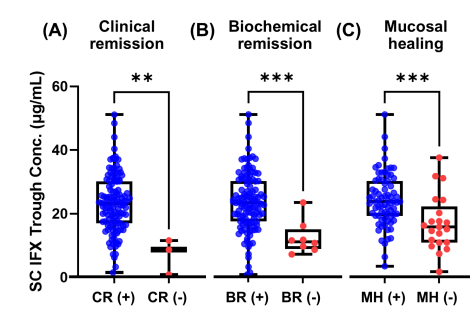
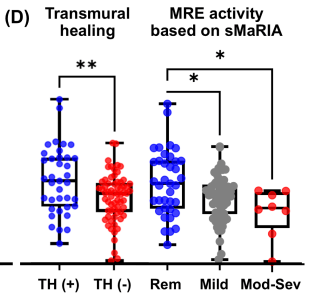
Discussion:
Target trough levels: In this study, the authors found that “the C-trough thresholds for clinical remission, biochemical remission, MH and TH were 12, 16, 18 and 30 μg/mL, respectively, based on ROC analysis. The C-trough of SC-IFX increased with the depth of remission.”
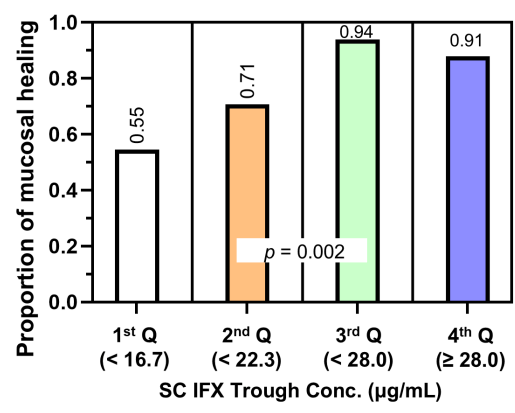
Why trough level targets may differ between IV administration and SC: Administration via the IV route results in early and rapid peak concentration followed by a steady decline to trough, whereas administration via the SC route has slower absorption, lower bioavailability, lower peak concentration and smaller differences between peak and trough concentrations.
The authors note that a study by Ye et al (United European Gastroenterology Journal; 2020: 8: 385–386) with 55 patients found that a C-trough >26.6 mcg/mL achieved clinical remission and fecal calprotectin levels <250 mcg/g at week 54 in 79% and 91% respectively compared to 46% and 62% in those with with C-trough <16.4 mcg/g.
These C-trough levels are significantly higher that the median C-trough levels of standard dosing (120 mg biweekly) in a phase 1 dosing RCT which was only 13.3 mcg/mL (S Schreiber et al. Gastroenterology 2018; 154: 1371). The dosing of 180 mg and 240 mg biweekly resulted in C-trough levels of 19.9 mcg/mL and 26.5 mcg/mL respectively.
My take: This study suggests that therapeutic drug monitoring will have different targets with SC-IFX than with IV-SC. SC formulations will offer more convenience. However, more effort will be needed to make sure patients are adherent with therapy in order to achieve optimal outcomes.
Related study: S. N. Hong, J. Hye Song, S. Jin Kim, et al. Inflammatory Bowel Diseases 30 (2024): 517–528. One-Year Clinical Outcomes of Subcutaneous Infliximab Maintenance Therapy Compared With Intravenous Infliximab Maintenance Therapy in Patients With Inflammatory Bowel Disease: A Prospective Cohort Study. In this prospective study with 61 patients, SC IFX switch induced a higher 1-year durable remission rate than continuing IV IFX in patients with IBD during scheduled maintenance therapy.
Related blog posts:
- LIBERTY Trials for Subcutaneous Infliximab
- Vedolizumab and Infliximab: Expected Dosing When Switching From IV to SC Routes
- FDA-Approved Subcutaneous Infliximab (Zymfentra) Now Available
- SC Infliximab versus Vedolizumab for Crohn’s Disease and for Ulcerative Colitis
- REMSWITCH: Infliximab IV to SC Study
Disclaimer: This blog, gutsandgrowth, assumes no responsibility for any use or operation of any method, product, instruction, concept or idea contained in the material herein or for any injury or damage to persons or property (whether products liability, negligence or otherwise) resulting from such use or operation. These blog posts are for educational purposes only. Specific dosing of medications (along with potential adverse effects) should be confirmed by prescribing physician. Because of rapid advances in the medical sciences, the gutsandgrowth blog cautions that independent verification should be made of diagnosis and drug dosages. The reader is solely responsible for the conduct of any suggested test or procedure. This content is not a substitute for medical advice, diagnosis or treatment provided by a qualified healthcare provider. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a condition.

