M Dubinsky et al. Adv Ther. 2023; 40(9): 3896–3911. Open Access! Matching-Adjusted Indirect Comparison Between Risankizumab and Ustekinumab for Induction and Maintenance Treatment of Moderately to Severely Active Crohn’s Disease
Background/Methods: Risankizumab (RZB) and ustekinumab (UST), interleukin (IL)-23 and IL-12/23 inhibitors, respectively, are approved treatments for moderately to severely active Crohn’s disease (CD); direct comparison between the two is ongoing. The authors indirectly compared efficacy of RZB versus UST using data from phase 3 trials (three trials for each medication):
- RZB: NCT03104413; NCT03105128; NCT03105102
- UST: NCT01369329; NCT01369342; NCT01369355
Key findings:
Induction: Higher proportions of patients achieved clinical and endoscopic outcomes with RZB vs. UST, resulting in significantly (p ≤ 0.05) greater percent differences between groups for CDAI remission (15%) and endoscopic response (26%) and remission (9%)
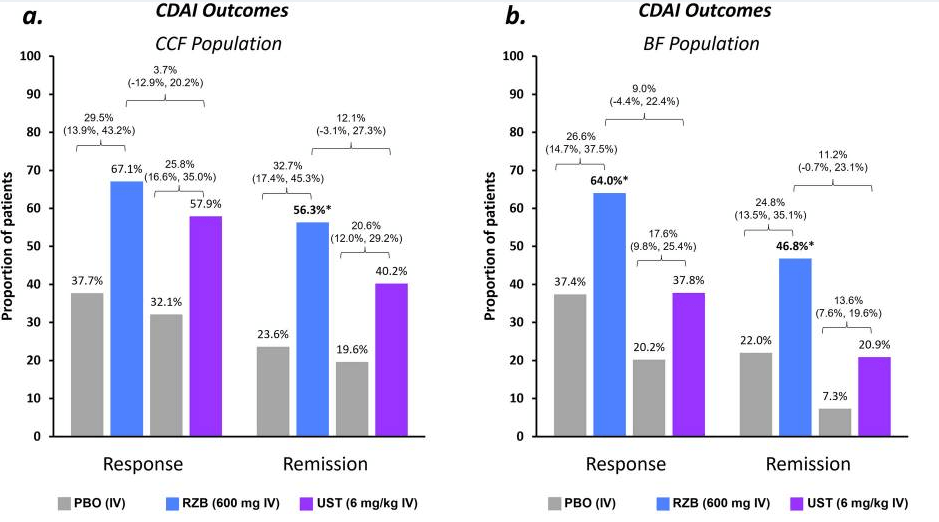
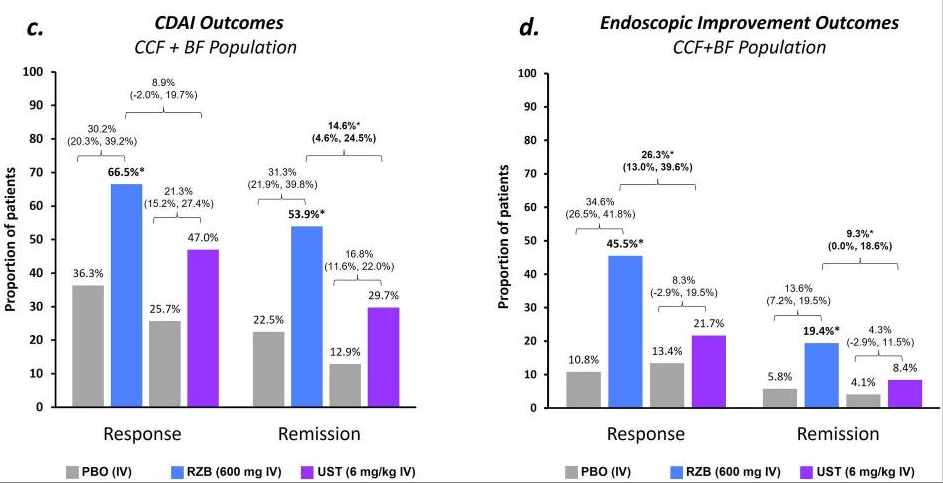
Maintenance: rates of CDAI remission were similar (range − 0.3% to − 5.0%) for RZB vs. UST; however, endoscopic response and remission rates appeared more favorable (see Figure 2 below)
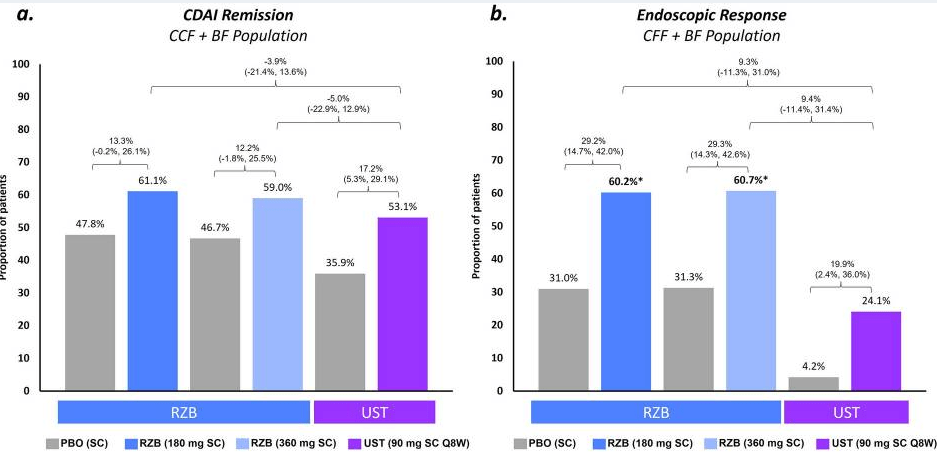
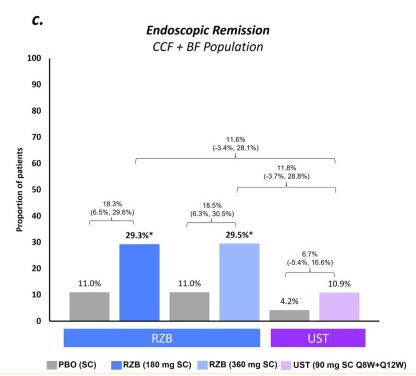
My take: Since this was not a direct randomization trial, these results are not definitive. However, in this indirect analysis, risankizumab appears to be superior to utekinumab in effectiveness of for Crohn’s disease.
Related blog posts:
- CCFA 2023 (Atlanta) Part 4
- CCFA 2023 (Atlanta) -Part 1
- How Much Ustekinumab (Stelara) Is Needed to Get a Good Response
- Risankizumab Receives FDA Approval for Crohn’s Disease (2022)
- Comparative Efficacy: Infliximab vs. Ustekinumab
- Which is a More Effective First-Line for Crohn’s Disease: Ustekinumab or anti-TNF agents?
- IBD Updates: Treat-to-Target Uptake, Long-Term Data on Ustekinumab Intensification, and Low Rates of C diff with Tofacitinib (& Clinical Pearl)
- CCFA 2023 (Atlanta) -Part 1
Disclaimer: This blog, gutsandgrowth, assumes no responsibility for any use or operation of any method, product, instruction, concept or idea contained in the material herein or for any injury or damage to persons or property (whether products liability, negligence or otherwise) resulting from such use or operation. These blog posts are for educational purposes only. Specific dosing of medications (along with potential adverse effects) should be confirmed by prescribing physician. Because of rapid advances in the medical sciences, the gutsandgrowth blog cautions that independent verification should be made of diagnosis and drug dosages. The reader is solely responsible for the conduct of any suggested test or procedure. This content is not a substitute for medical advice, diagnosis or treatment provided by a qualified healthcare provider. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a condition.

