Over the next 2 weeks or so, I am posting my notes/pictures from this year’s annual meeting. The first few days will review the postgraduate course. For the most part, I find the postgraduate course reassuring that I have kept up with current approaches; there is usually not a lot of new information but a solid review of the topics.
Here is a link to postgraduate course syllabus: NASPGHAN PG Syllabus – 2017
This blog entry has abbreviated/summarized these presentations. Though not intentional, some important material is likely to have been omitted; in addition, transcription errors are possible as well.
Strictures beyond the esophagus
Petar Mamula, Children’s Hospital of Philadelphia
Some useful points:
- Fluoroscopy very useful with most strictures –may improve safety and effectiveness. Helps define anatomy
- Reviewed strictures in stomach –rare. May be due to caustic ingestion, Crohn’s disease or chronic granulomatous disease
- Intestinal/colonic strictures (or narrowing): duodenal webs -can be treated with needle knife, Crohn’s disease strictures -can be balloon dilated, Short gut syndrome, Graft versus host disease
GI Bleeding Update
Diana Lerner Medical College of Wisconsin
Useful points
Upper GI Bleeding:
- IV PPIs reduce risk of transfusion and reduce risk of re-bleeding
- IV PPI BID treatment has been shown to be noninferior to continuous drip
- Conservative transfusion therapy
- Erythromycin can be helpful
- Lecture had good videos with review of techniques: clipping, heater probe, epinephrine injection (not recommended as monotherapy), argon plasma coagulation, and bipolar electrocautery
Lower GI Bleeding:
- Etiologies include the followiing: Post-polypectomy, Solitary Rectal Ulcer syndrome, Blue Rubber Bleb syndrome, anastomotic ulcer bleeding, Meckel’s diverticulum
- Lower GI evaluation is best after prep –much higher yield
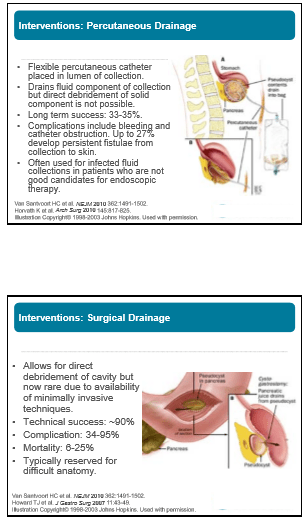
Management of Pancreatic Fluid Collections
Matt Giefer Seattle Children’s Hospital
Key points:
- Imaging in first 7 days of diagnosis may miss the development of fluid collections
- With necrotizing pancreatitis, fluid collections are either ANC: acute necrotic collection (<4 weeks) or WON: walled off necrosis (>4 weeks); Bryan et al. Radiographics 2016; 36: 675
- With interstitial edematous pancreatitis, fluid collections are either acute peripancreatic fluid collection (<4 weeks) or Pseudocyst: >4 weeks,
- Fluid collections do not preclude feeding patients
- Drainage often needed if fluid collection becomes infected or if fluid collection causes obstruction
- Endoscopic drainage is first-line approach: equally effective as surgery, fewer complications, equal efficacy, and lower cost