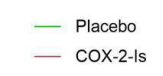T Guru et al. Gastroenterology 2025: 169:1499 – 1509. Total Pancreatectomy With Islet Autotransplantation for Chronic Pancreatitis
This prospective multicenter study included 384 patients with a mean age of 30 years (34% pediatric).
Key findings:
- Daily abdominal pain decreased from 65% to 23%, whereas the mean pain score decreased from 4.9 (SD, 2.3) to 2.3 (SD, 2.5; both P < .001)
- Opioid use decreased (assessed over a 14-day interval) from 61% before to 24% at 1 year after TPIAT (P < .001)
- Improved physical and mental health: Physical component summary and mental component summary scores improved by ≥10 points in 58% and 35%, respectively
- Mean hemoglobin A1c was 7% (SD, 1.9%) with 20% insulin independent at 1 year
- Young age was associated with better outcomes, whereas duration and etiology of disease did not predict response to TPIAT
In their discussion, the authors note that it had been “widely hypothesized that after several years of pain, mechanism shift to solely neuropathic pain, such that surgery would be unlikely to offer benefit. Our results suggest this is not true.”
My take: This study provides robust data supporting TPIAT as average pain scores declined by more than 50%, the need for opioid analgesics decreased substantially, and mental health/physical health/QOL all improved. Most maintained glycemic control.
Related blog posts:
- NASPGAN Paper: Surgery for Chronic Pancreatitis: Choose Your Operation and Surgeon Carefully
- Total Pancreatectomy with Islet Autotransplantation for Refractory Recurrent Pancreatitis
- How to Upgrade Pancreas Care –Jay Freeman MD (Part 1)
- How to Upgrade Pancreas Care –Jay Freeman MD (Part 2)
- Pancreatitis Update (Part 2) (2017)
Humor (from 2009): YouTube Link: Brain Surgeon – That Mitchell & Webb Look (2:09 min)