MG Stahl et al. Gastroenterol 2026; 170: 240-245. Open Access! Mass Screening of Celiac Disease: A Crossing Point Between Secondary and Primary Prevention?
The authors make several good arguments for mass screening for celiac disease.
Key Points:
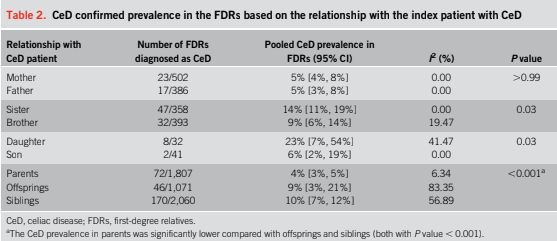
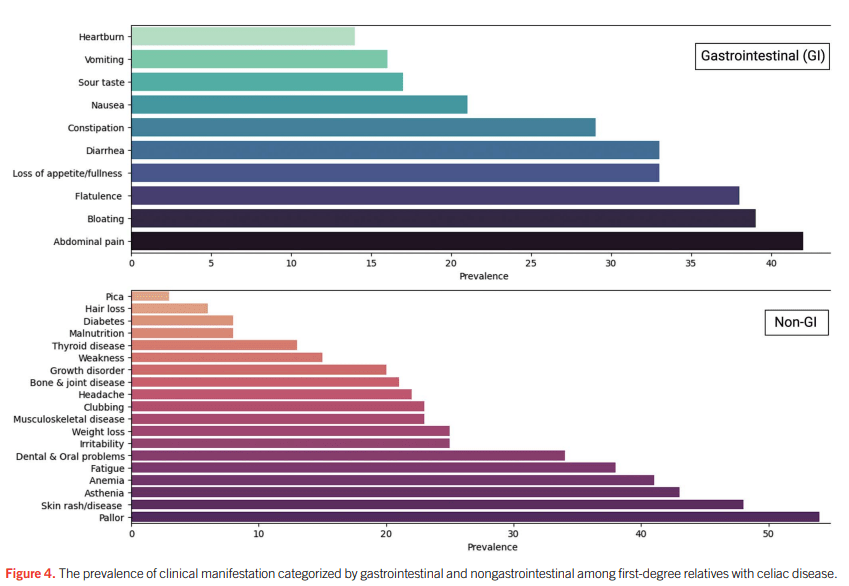
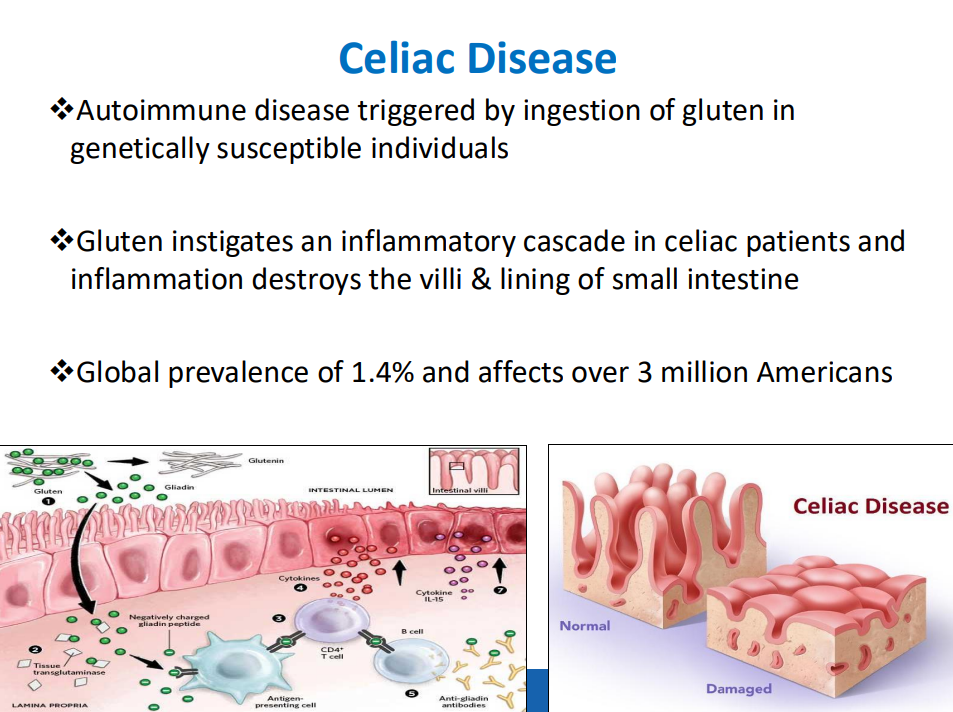
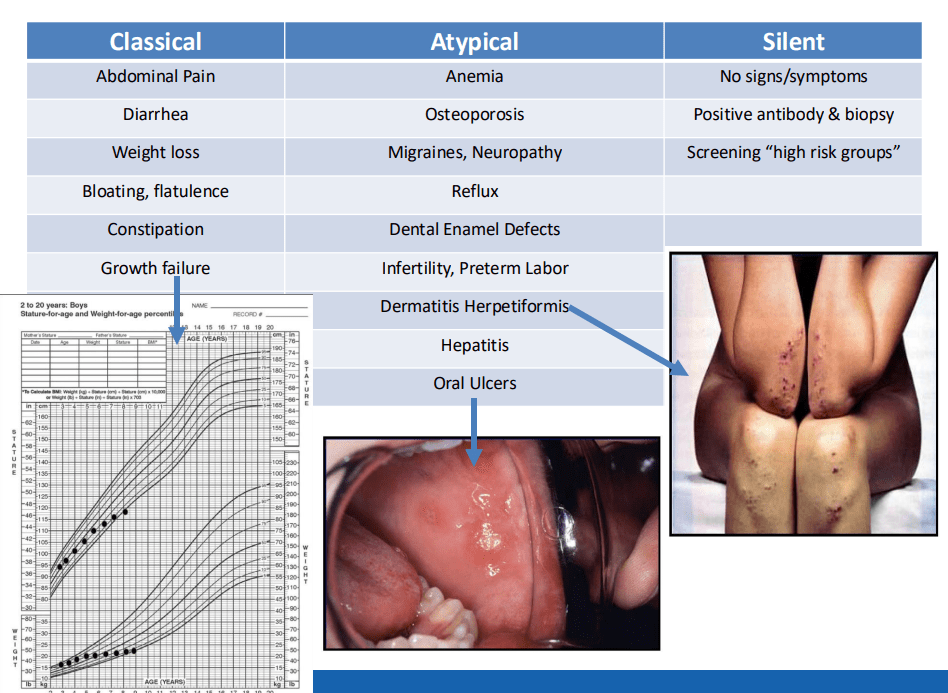
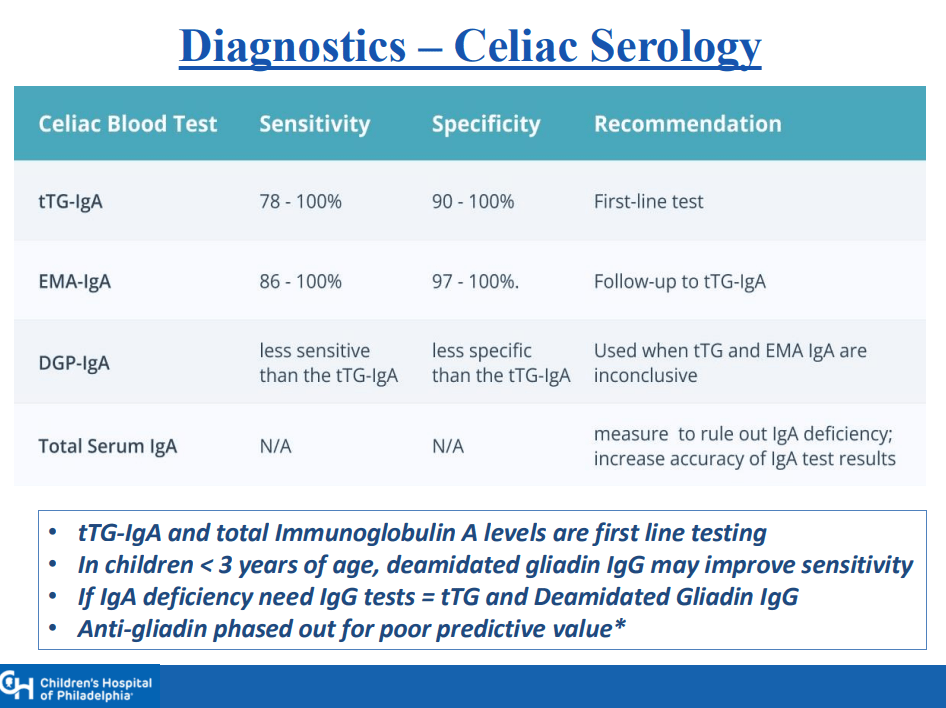
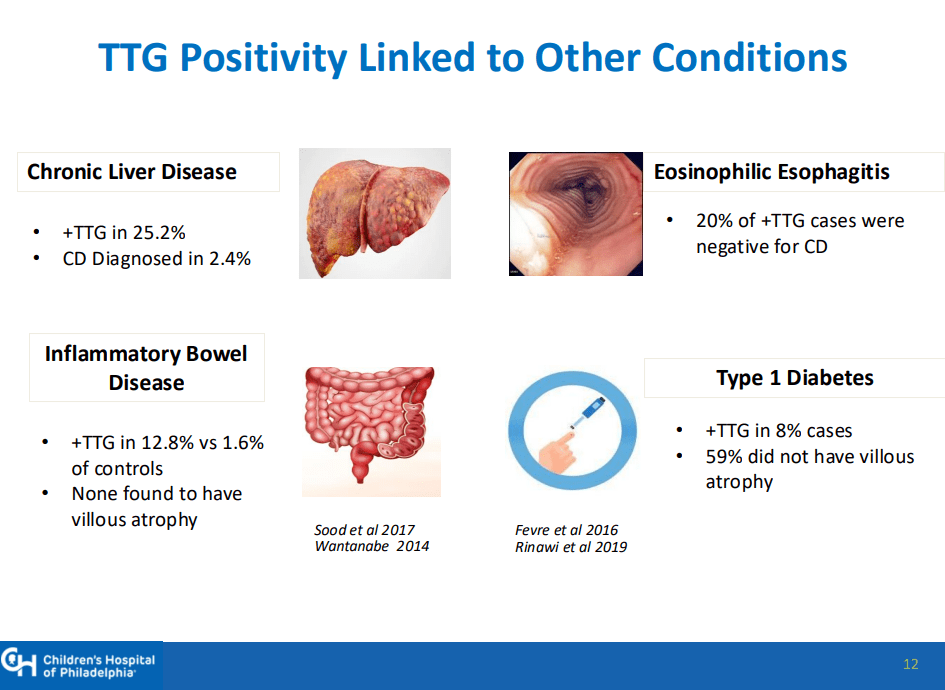
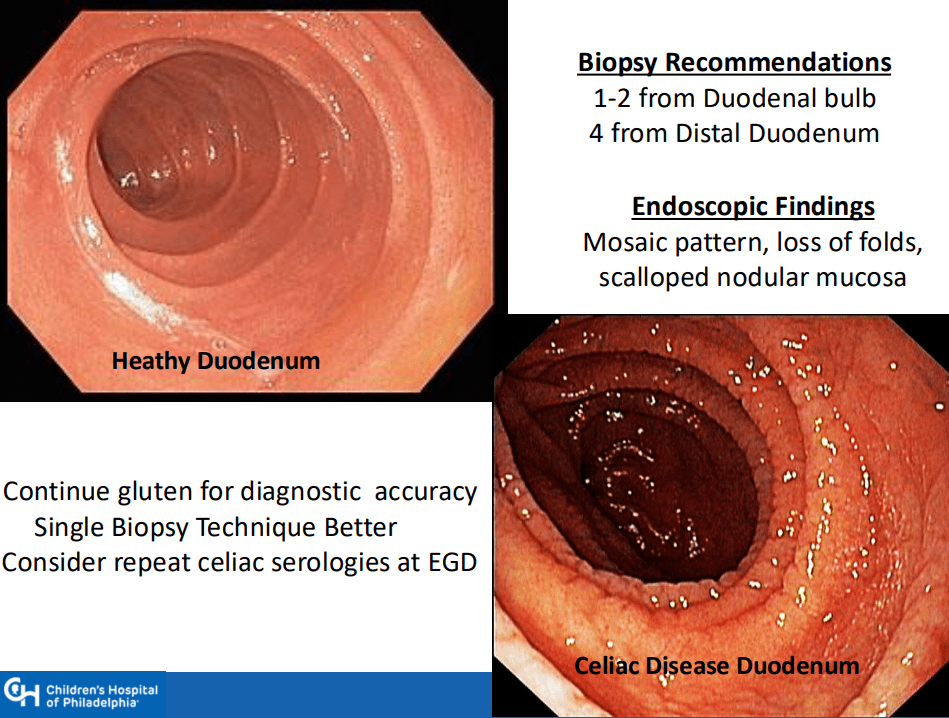
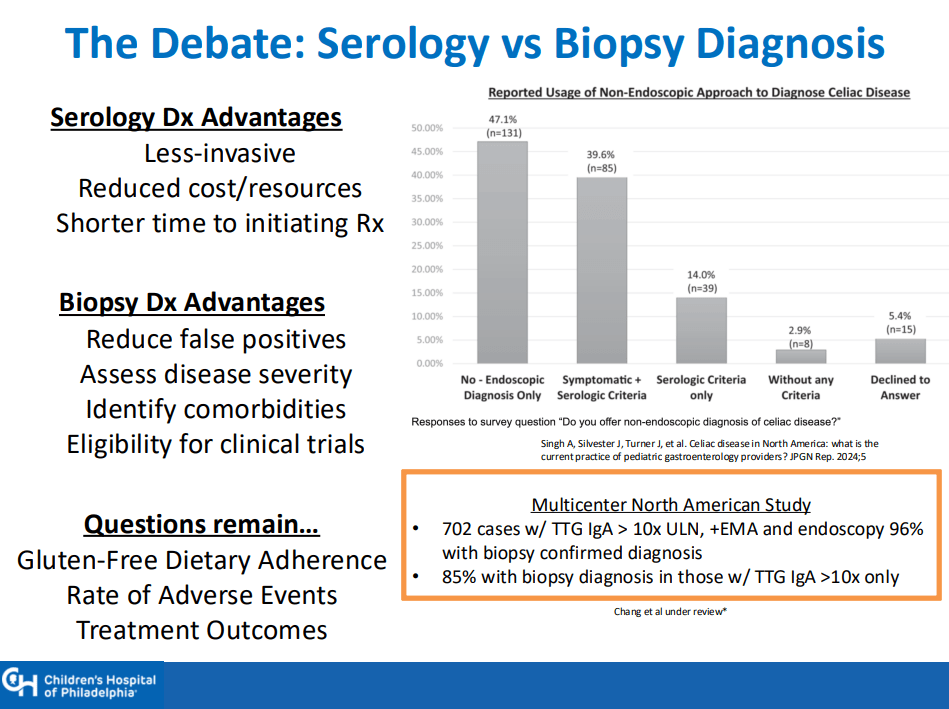
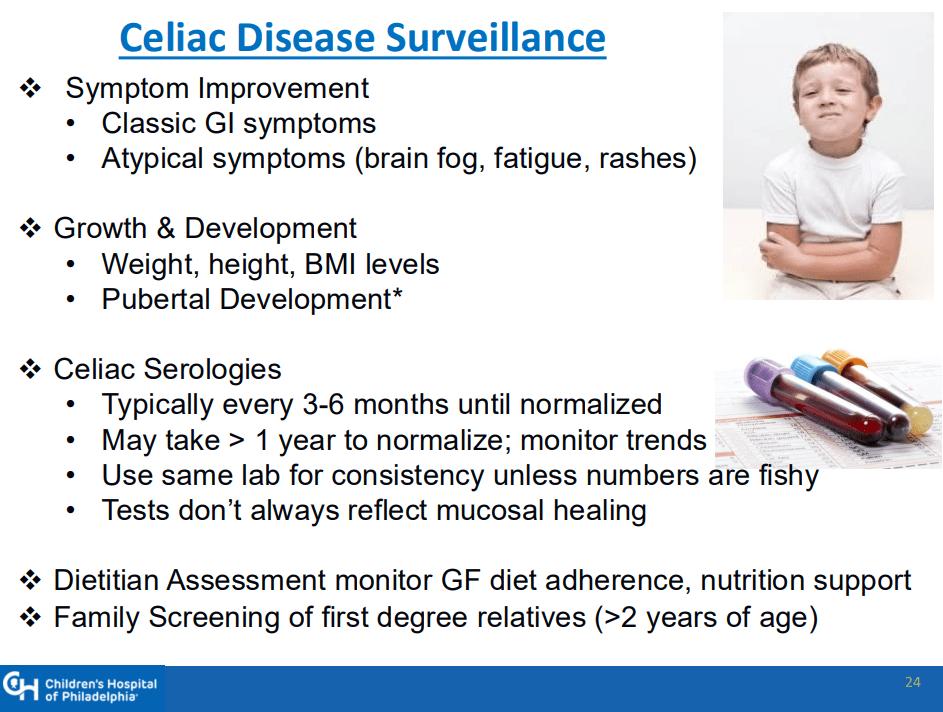
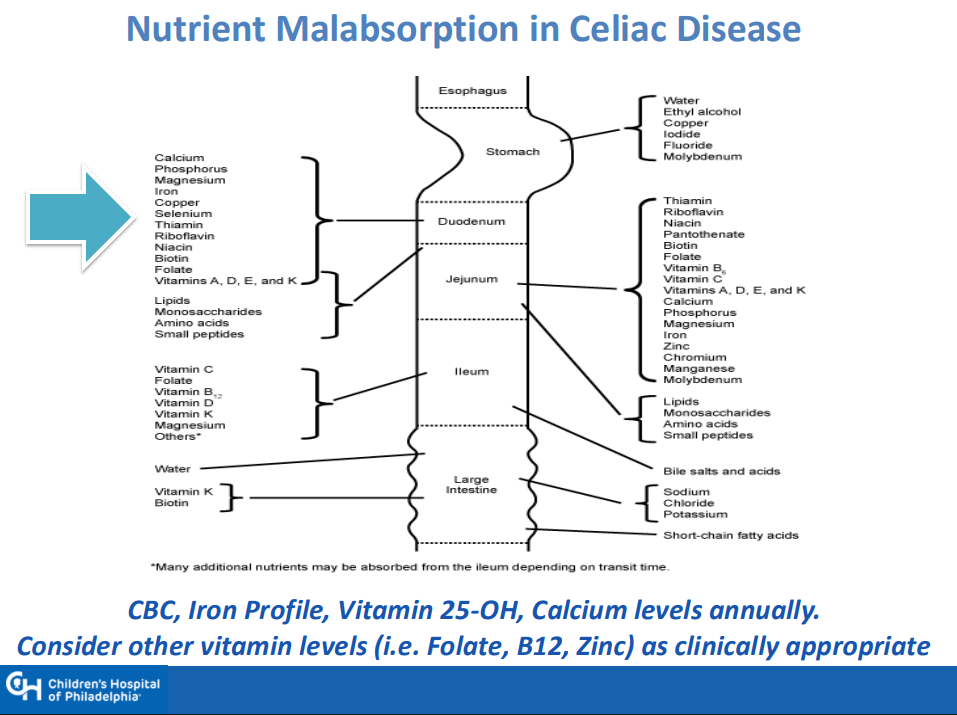
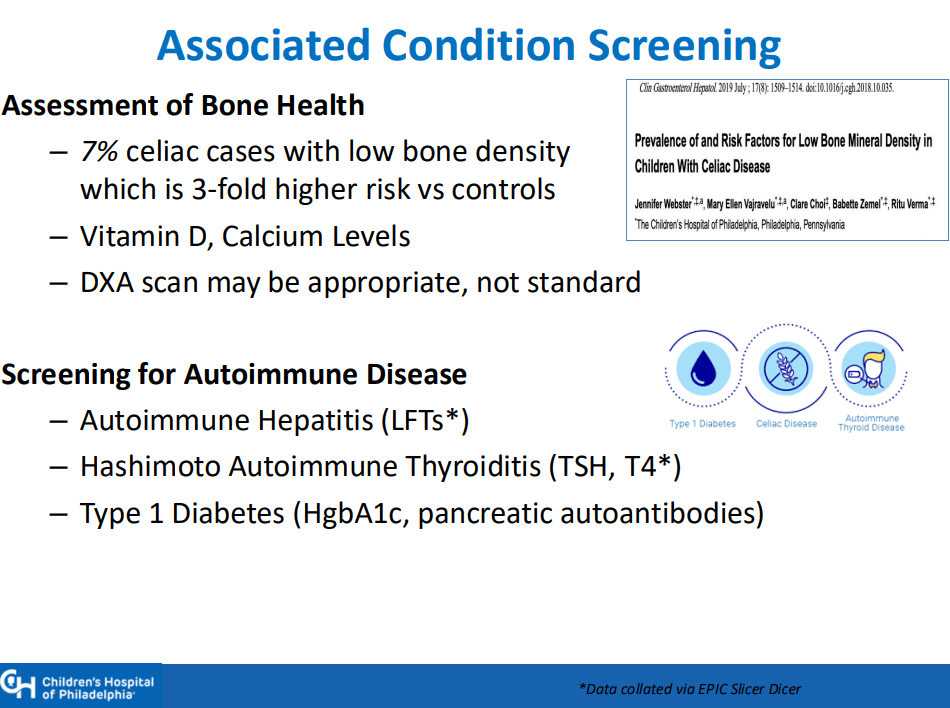
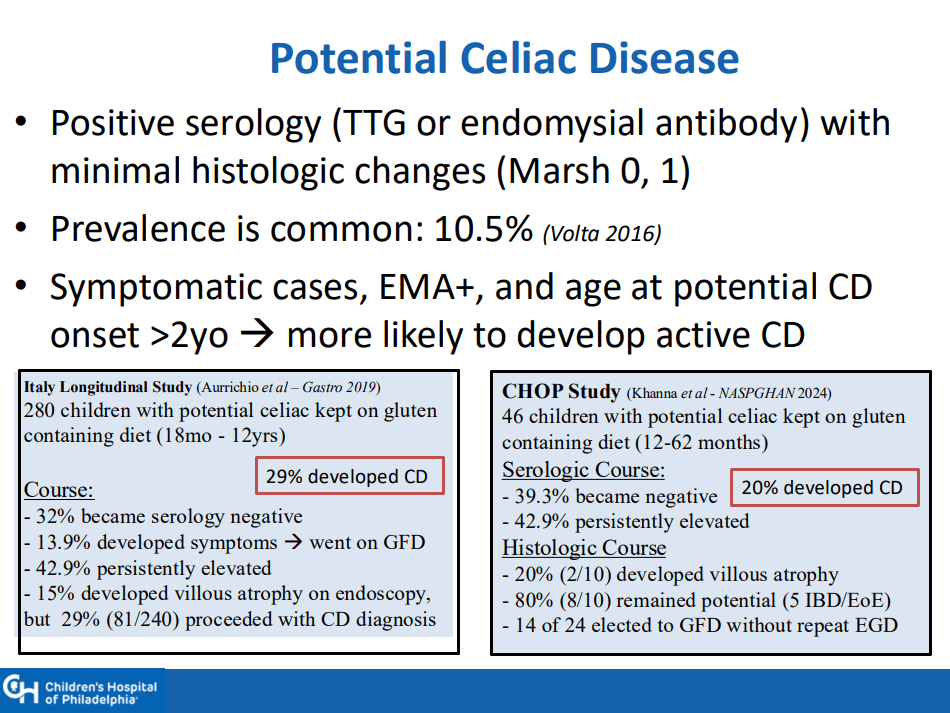
- “The worldwide prevalence is high, affecting an estimated 1% to 3% of the general population…One study showed that two-thirds of children remain undiagnosed.” (Dig Liver Dis. 2023; 55:608-613). Thus, screening would identify many cases that would otherwise go undiagnosed and untreated
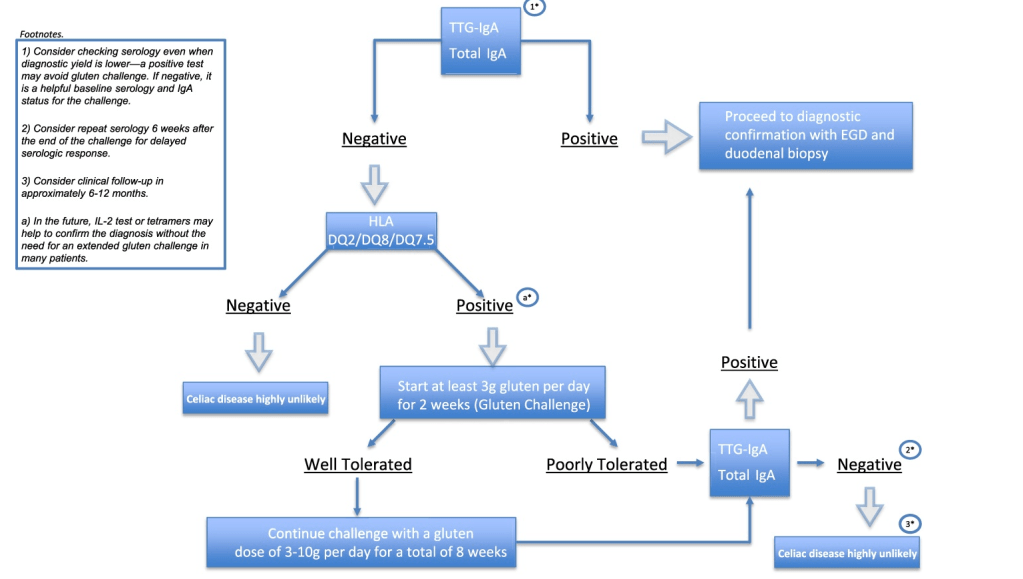
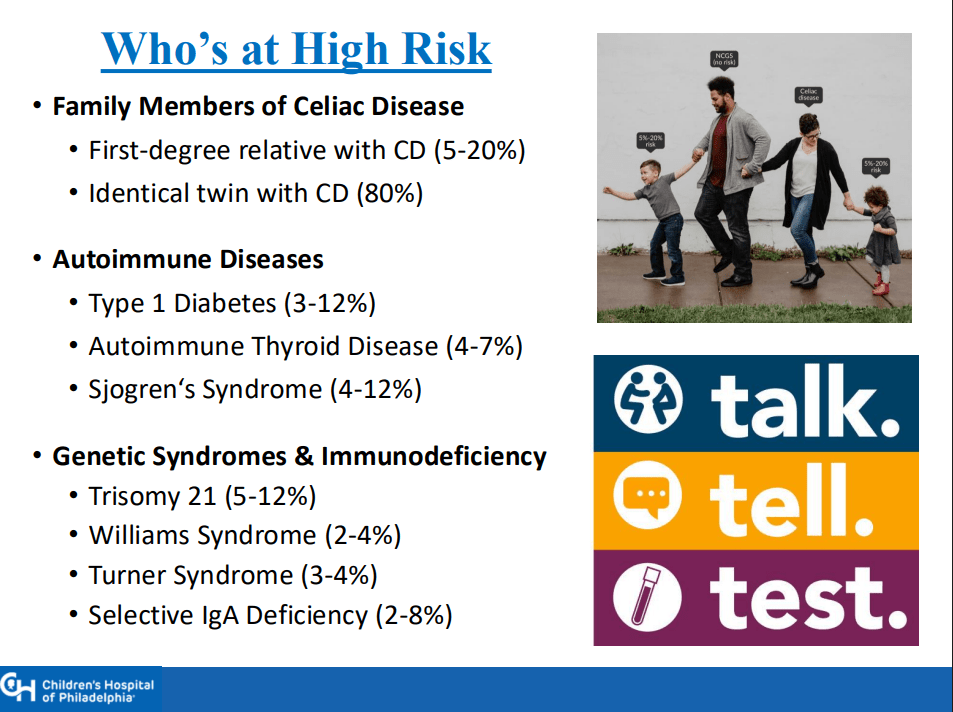
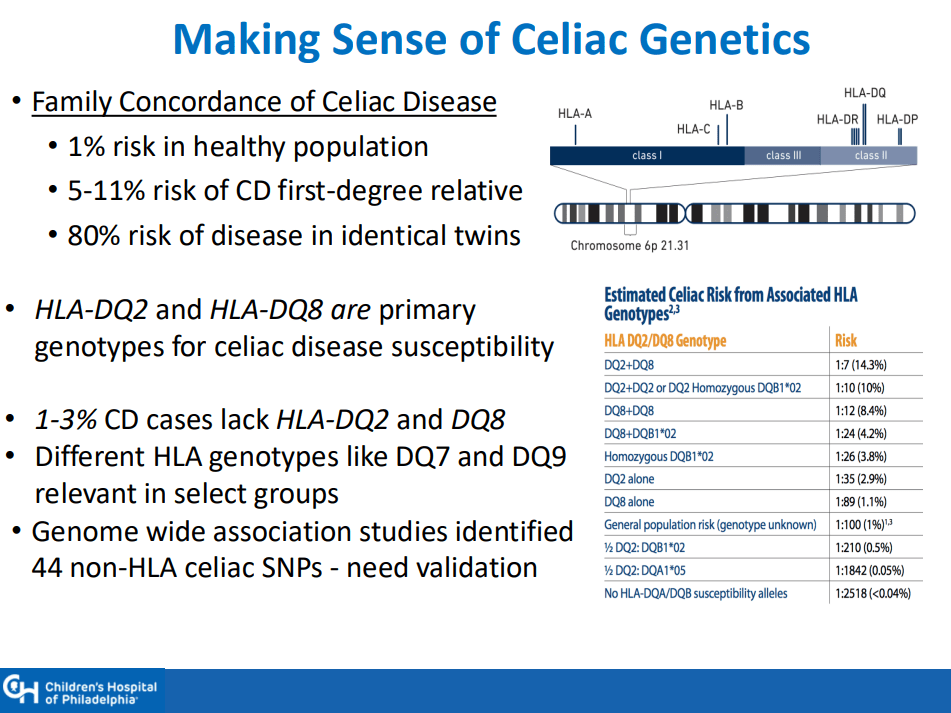
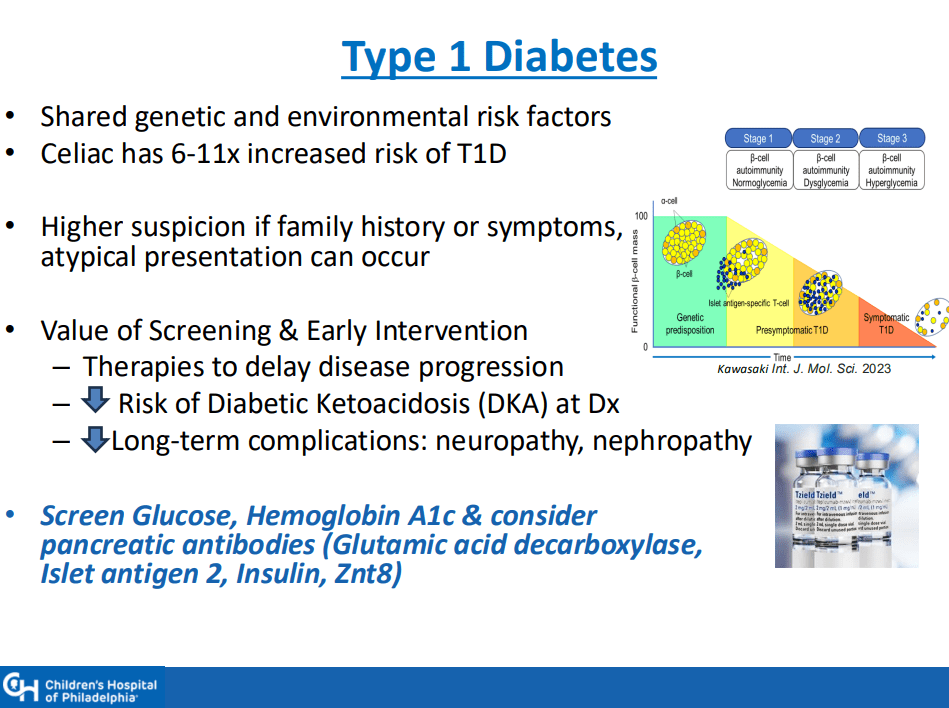
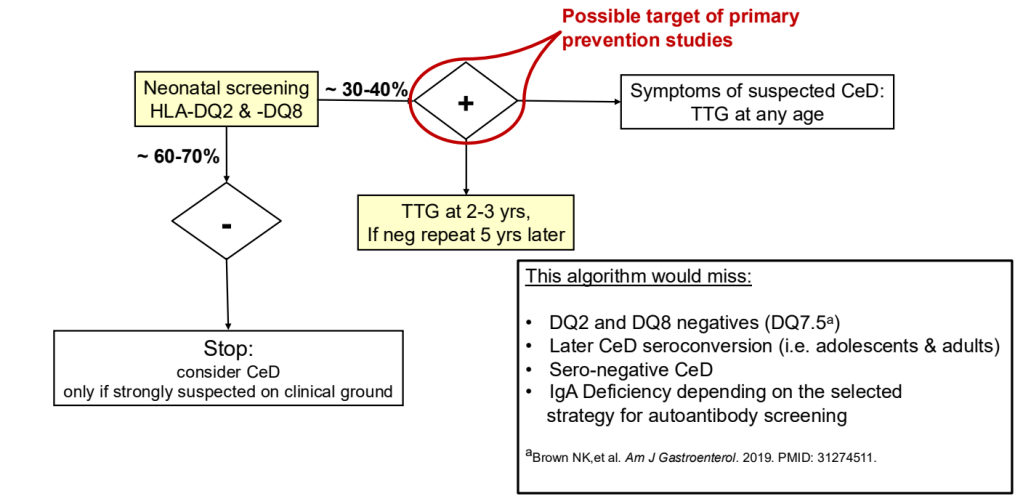
- ” As one of the most HLA-restricted diseases, nearly all patients carry HLA-DQ2.5, DQ2.2, or DQ8 (and very rarely DQ7.5), and their absence makes CeD extremely unlikely. Although 30% to 40% of the general population carry these alleles, only approximately 3% will go on to develop CeD”
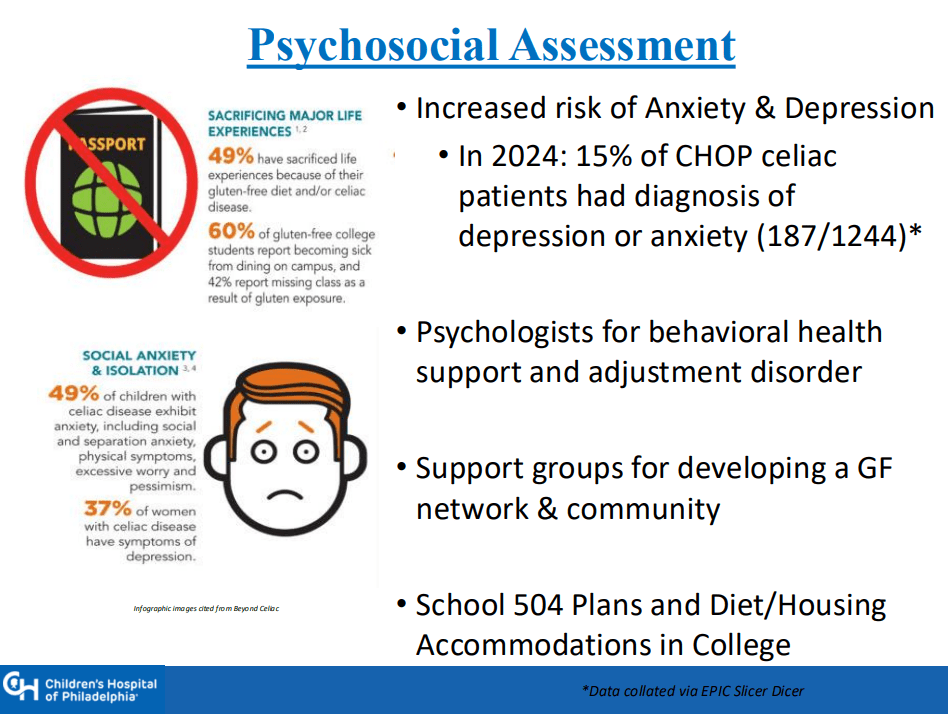
- “The Environmental Determinants of Diabetes in the Young (TEDDY) study showed that although there was some early anxiety reported, there was no long-term psychological harm in disclosing genetic risk to parents of children that were tested and most parents adapted over time”
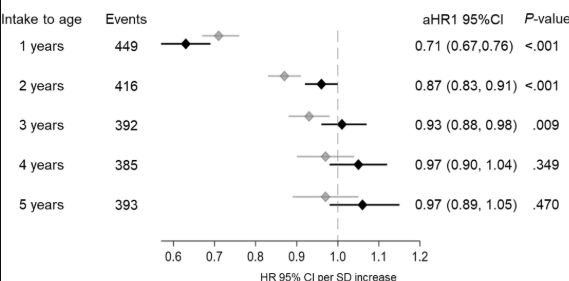
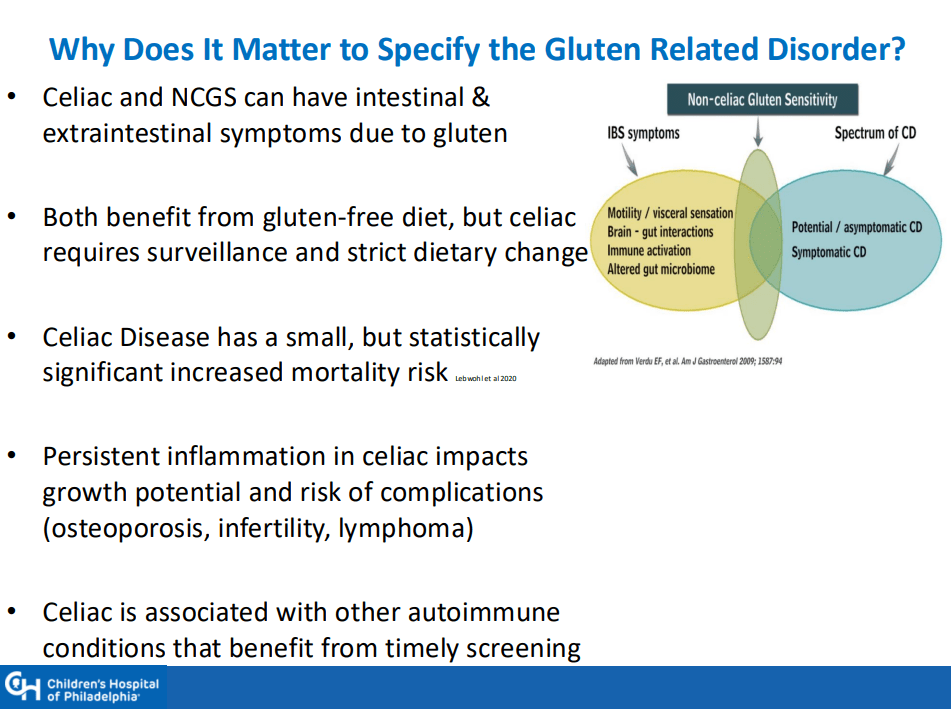
- “Children identified as highest risk at birth through HLA-DQ typing also represent an ideal cohort to test interventions such as dietary modifications or microbiome targeted therapies during the predisease phase, during the key “window of opportunity,” ideally before seroconversion (primary prevention).” There are no currently proven primary prevention interventions.
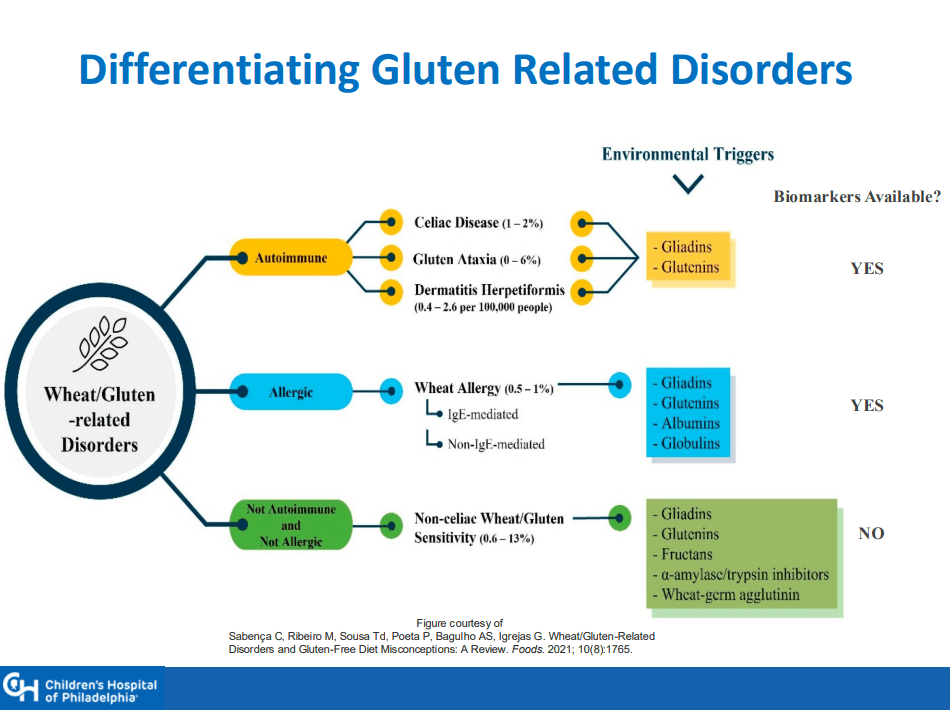
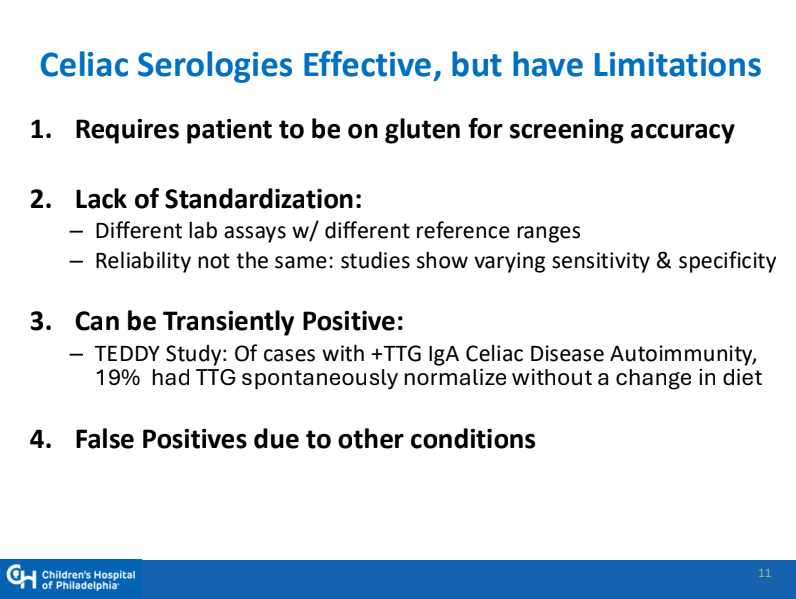
- Some of the drawbacks to screening: 1. “Evidence on health benefits of treating asymptomatic CeD is limited.” 2. “The potential psychosocial effects of both newborn genetic testing and a strict gluten-free diet in individuals diagnosed through screening need to be considered.” 3. “HLA-DQ typing may be cost prohibitive in some regions of the world.” 4. “Most children identified to be at risk for CeD based on HLA-DQ will not develop CeD.”
My take: I am skeptical about the benefits of screening at birth and how it would work in our current health care system. We have plenty of examples in our field in which early screening is not followed up well (eg. Hepatitis C, Biliary Atresia). If more evidence emerges on the benefits of primary prevention, then more widespread screening at birth may be worthwhile. For example, there is “a clinical trial underway in Sweden, the GRAin study (NCT04593888) that aims to investigate whether eating a gluten reduced diet (<2 g of gluten per day) may reduce the risk of CeD in children with genetic risk.”
Related blog posts:
- Missing Opportunities to Cure Pediatric Hepatitis C Infection
- Don’t Put the Cart Before the Horse: Biliary Atresia Screening
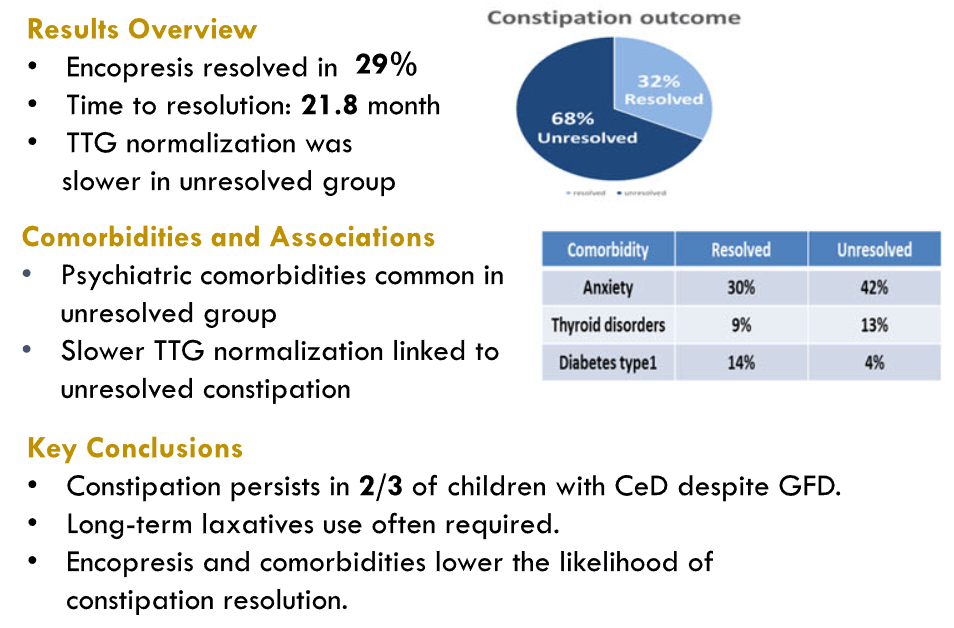
- Outcomes in Children with Celiac Disease Presenting with Constipation
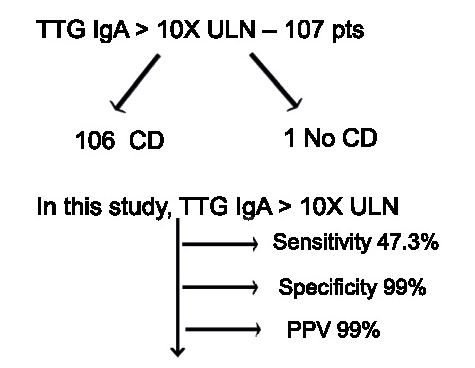
- Likelihood of Celiac Disease with Conflicting Serology Results
- Shared Decision-Making in Celiac Disease Diagnostic Approach
- When Celiac Disease Symptoms Continue Despite a Gluten Free Diet
- Celiac Studies: Lower Rates of Undiagnosed Celiac Disease in Norway, and Lower Rates of Celiac with Early Dietary Fiber
- Dr. Arun Singh: Tips and Tricks to Managing Celiac Disease