A Hart et al. Gastroenterol 2025 (ePUB Ahead of Print) Open Access! Efficacy and Safety of Guselkumab Subcutaneous Induction and Maintenance in Participants With Moderately to Severely Active Crohn’s Disease: Results From the Phase 3 GRAVITI Study
Background: “Guselkumab is a selective dual-acting IL-23p19 subunit inhibitor that potently neutralizes IL-23 by binding to the p19 subunit and to CD64, a receptor on cells that produce IL-23…In the double-blind Phase 2 GALAXI 1 study and the 2 identically designed double-blind Phase 3 GALAXI 2 and GALAXI 3 studies, guselkumab intravenous (IV) induction (200 mg at weeks 0, 4, and 8) followed by subcutaneous (SC) maintenance (200 mg every 4 weeks or 100 mg every 8 weeks) demonstrated efficacy compared with placebo… In addition, guselkumab demonstrated superiority to ustekinumab for multiple endoscopic-based endpoints at week 48 in pooled data from GALAXI 2 and GALAXI 3… The GRAVITI study…evaluated the efficacy and safety of guselkumab SC induction followed by SC maintenance in participants with moderately to severely active Crohn’s disease.
Methods: This was a Phase 3 double-blind, placebo-controlled, treat-through GRAVITI study randomized 347 participants 1:1:1 to guselkumab 400 mg SC every 4 weeks→100 mg SC every 8 weeks (n = 115), guselkumab 400 mg SC every 4 weeks→200 mg SC every 4 weeks (n = 115), or placebo (n = 117). Placebo participants meeting rescue criteria received guselkumab from week 16 onward.
Key Findings:
- At week 12, significantly greater proportions of participants receiving guselkumab 400 mg achieved clinical remission vs placebo (56.1% vs 21.4%; P < .001), and endoscopic response vs placebo (41.3% vs 21.4%; P < .001)
- At week 48, significantly greater proportions of participants in both guselkumab groups (100 mg SC every 8 weeks: 60.0%; 200 mg SC every 4 weeks: 66.1%) achieved clinical remission vs placebo (17.1%; P < .001 each) and endoscopic response (44.3%; 51.3%; vs placebo 6.8%; P < .001 each)
- Immunogenicity: “Antibodies to guselkumab were detected in 24 (8.8%) of the 274 guselkumab-treated participants through week 48. Only 3 of 274 participants (1.1% of the total population) were positive for neutralizing antibodies. Through week 48, no impact of antibodies to guselkumab on serum guselkumab concentrations, efficacy, or injection-site reactions was observed”
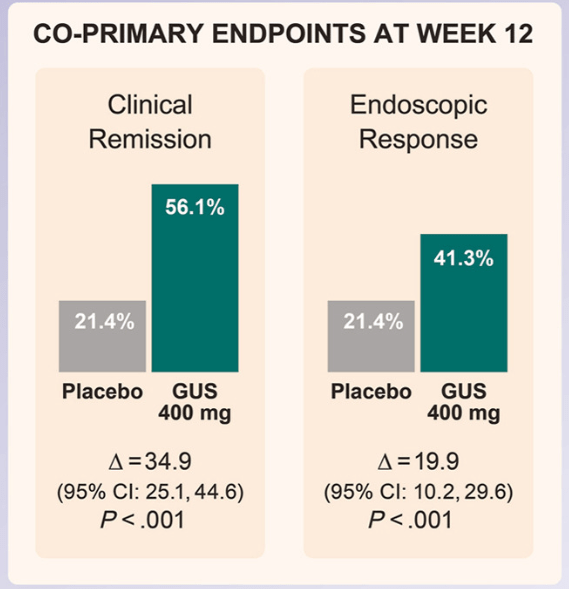
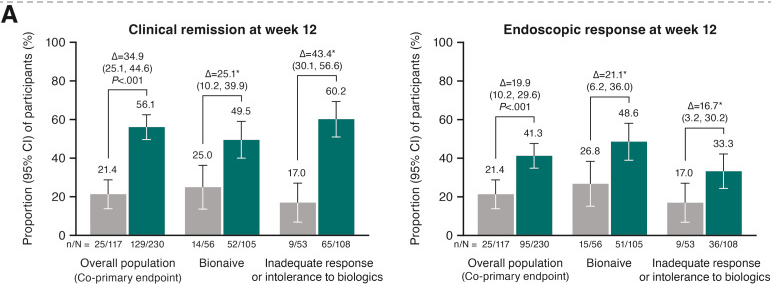
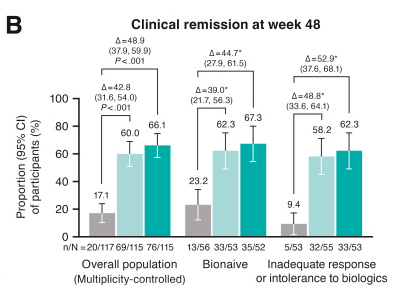
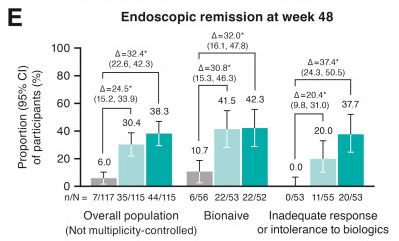
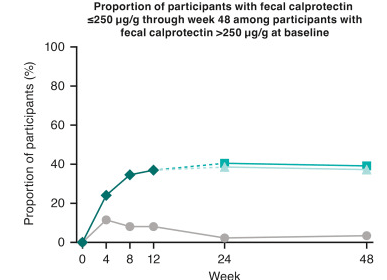
Discussion: “The results presented here from GRAVITI were consistent with those reported in the double-blind, treat-through GALAXI trials in which guselkumab induction was administered IV in participants with moderately to severely active Crohn’s disease. For example, 41.3% of participants in the GRAVITI study achieved endoscopic response 12 weeks … whereas 36.9% of participants in the pooled GALAXI studies achieved endoscopic response 12 weeks after guselkumab… IV induction (placebo: 12.2%).”
My take: This study shows that Guselkumab with a SC induction is safe and effective in participants with moderately to severely active Crohn’s disease. IV induction does not appear to be needed. Though IL-23 agents have been important advances, there are still a large number of patients without a good response.
Related blog posts:
- Guselkumab: Expanding the GALAXI of Treatments for Crohn’s Disease
- Pivotal Study: Guselkumab Efficacy in Ulcerative Colitis (QUASAR study)
- IBD Updates: Insurance Barriers Hindering Care, Guselkumab vs Ustekinumab, IBD Pain Management Guidelines
- Dr. Maria Oliva-Hemker: Positioning Therapies for Pediatric Crohn’s Disease