KWE Sweerts et al. Alimentary Pharmacology & Therapeutics, 2025; 61:702–705. Open Access! Analysis of Antroduodenal Motility in Patients With Hypermobility Spectrum Disorders/Hypermobile Ehlers–Danlos Syndrome
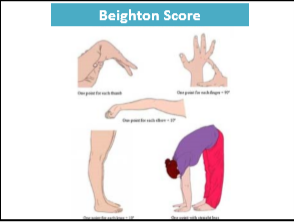
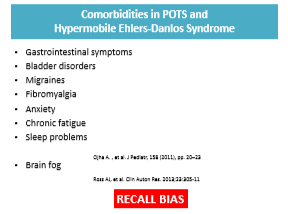
Background: Hypermobility spectrum disorders (HSD) and hypermobility Ehlers–Danlos syndrome (hEDS) are frequently associated with gastrointestinal symptoms, although the underlying mechanisms remain unclear. Since recruitment occurred before the 2017 criteria for hEDS were established, it was not possible to distinguish between HSD and hEDS.
Methods: Retrospective review of all patients (>18 yrs) referred t for gastrointestinal motility evaluation and undergoing ADM were consecutively included from 2009 to 2023. This included 239 patients (50 HSD/hEDS and 189 non-HSD/hEDS). The HSD/hEDS group showed a lower BMI and higher use of enteral feeding than the control group (p < 0.001 and p = 0.026, respectively). This group was also younger, with a mean age of 30.4 ± 11.1 years versus 45.3 ± 15.4 years (p < 0.001).
Key findings:
- The prevalence of antroduodenal dysmotility was not different between both groups, but enteric dysmotility was less common in the HSD/hEDS group (13% vs. 34%, p = 0.006).
- There were similar percentages of delayed gastric emptying than non-HSD/hEDS patients; delayed gastric emptying was highly prevalent in both groups, 85% in patients with HSD/hEDS and 94% in non-HSD/hEDS patients
- There were no differences in predominant symptoms between patients with and without HSD/hEDS.
In the discussion, the authors note that the lower rate of dysmotility combined with higher rates of enteral nutrition indicate that “factors like visceral hypersensitivity and autonomic function could be relevant in this context.”
My take: Most patients at this referral center had delayed gastric emptying. However, Ehlers-Danlos patients, in fact, had lower rates of enteric dysmotility.
Related blog posts:
- Myth or Fact: Joint Hypermobility is Related to Pediatric Functional Abdominal Pain & Dr. Roy Link
- Joint Mobility -Not Associated with Increased Functional GI Disorders