A Porto et al. J Pediatr Gastroenterol Nutr. 2025;81:5–10. New international infant formulas in the United States: Understanding the Food and Drug Administration-enforcement discretion
Background: In February 2022, the United States experienced a significant infant formula shortage, due to a major product recall by the country’s largest infant formula manufacturer, compounded by global supply chain issues and import restrictions.1, 2 In response, the Food and Drug Administration (FDA) launched Operation Fly Formula in mid-2022, which allowed international infant formula manufacturers to market, import and distribute their formulas in the United States…Currently, a total of five companies, who produce 14 international formulas, have opted to work with the FDA in transitioning to the US market.5 Many of these international formulas are significantly cheaper than the domestic alternatives, which have contributed to their rising popularity.
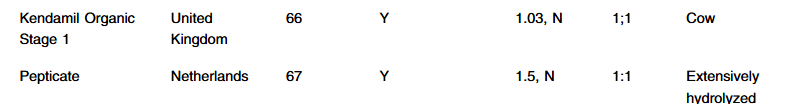
Key points:
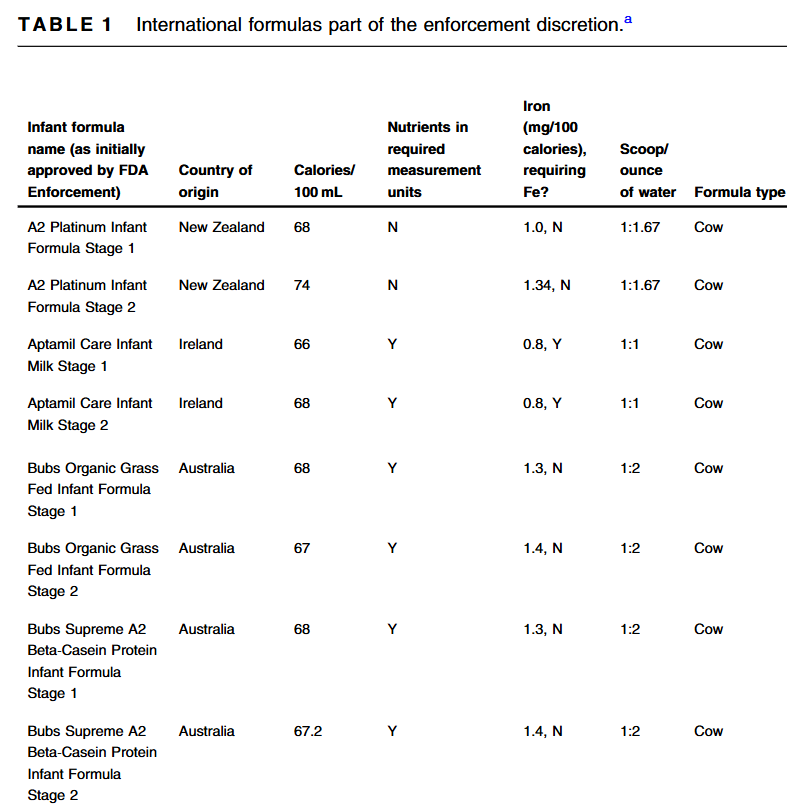
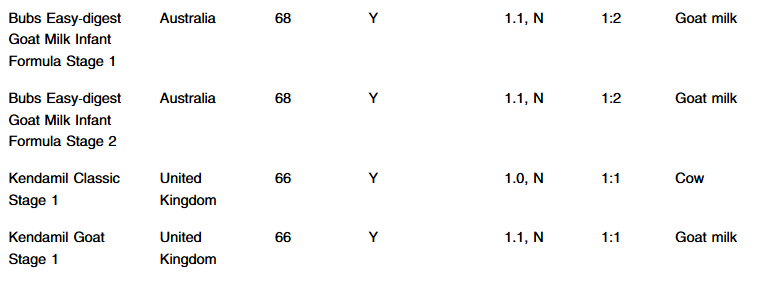
- 8 of 14 formulas are stage formulas with Stage 1 for 0-6 months, and Stage 2 for >6 months. “Stage 1 formulas tend to contain less iron, which may provide an insufficient amount of iron for infants >6 months.11 Also, infants <6 months should not consume Stage 2 formula since it does not contain carnitine, believed to be an essential nutrient in this age group.12“
- Of the 14 formulas, all the labels were in English and contained all the FDA nutrient requirement
- “Two of the imported formulas [Aptamil brands] contained less than 1 mg/100 calories of formula of iron, the minimum amount to be considered iron fortified by the FDA, and did include a label which highlighted that additional iron may be necessary”
- “All the foreign formulas contained prebiotics… The FDA, however, reports that probiotics can be dangerous for preterm infants and put them at risk for potentially fatal infection caused by the bacteria or yeast contained in the probiotic.6 Therefore, pediatricians should be aware that international formulas should not be used for preterm infants.”
- MIXING INSTRUCTIONS: “Eleven out of the fourteen international formulas use a different scoop to water ratio from what is typically standard of American formulas…coops from international formulas may also be a different size compared to their US counterparts. Given the variation in different mixing ratios and scoop sizes, there is a risk of formula being mixed incorrectly”
- “Consider that the family may be purchasing from a 3rd party vendor and ask for the specific website that they are purchasing from. Formulas should not be purchased at 3rd party vendor websites due to them being unregulated, and safety concerns with improper shipping or storage”
- “If the label is not in English, it is highly likely that the formula has been purchased through a 3rd party vendor. Recommend counseling on safety concerns as listed above. Many of the foreign infant formulas use different mixing ratios so it is important that parents read the label to confirm mixing ratios”
My take: The availability of FDA-approved international formulas has been helpful especially with recent shortages. This article makes several important points to assure their proper use, especially regarding mixing instructions and using Stage formulas for appropriate age.
Related blog posts: