- Gina Kolata, NY Times, 3/10/25: Mutated DNA Restored to Normal in Gene Therapy Advance
- Beam Therapeutics Press Release 3/10/25: Positive Initial Data for BEAM-302 in the Phase 1/2 Trial in Alpha-1 Antitrypsin Deficiency (AATD)
An excerpt from NY Times:
Researchers have corrected a disease-causing gene mutation with a single infusion carrying a treatment that precisely targeted the errant gene.This was the first time a mutated gene has been restored to normal….
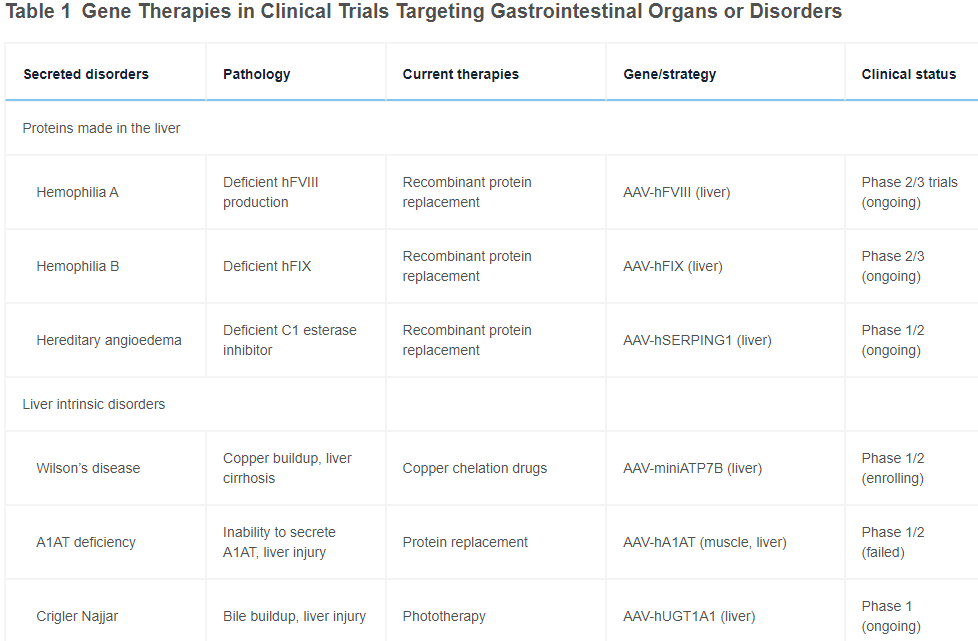
The study involved patients who have alpha-1 antitrypsin deficiency, or AATD, a genetic disease that affects an estimated 100,000 Americans…
When the nanoparticles reached the liver, the lipid layer peeled off, releasing the editor — a disabled CRISPR molecule that acted like a GPS for the genome and an enzyme to fix the mutation. The CRISPR molecule crawled along the patient’s DNA until it found the one incorrect letter that needed to be repaired among the three billion DNA letters in the genome. Then the editing enzyme replaced that letter with the correct one… Those who got the highest dose made enough normal alpha-1 antitrypsin to be in a range where no more damage should occur.

My take: This is exciting news, though, long-term data is needed to determine if this will be a durable cure. Cost/availability will be an important consideration if effective.
Related blog posts: