More from Aspen Webinar 2021. This blog entry has abbreviated/summarized several presentations. Though not intentional, some important material is likely to have been omitted; in addition, transcription errors are possible as well. An excellent review from Dr. Sokol.
What’s New with IFALD Ronald Sokol
Key points:
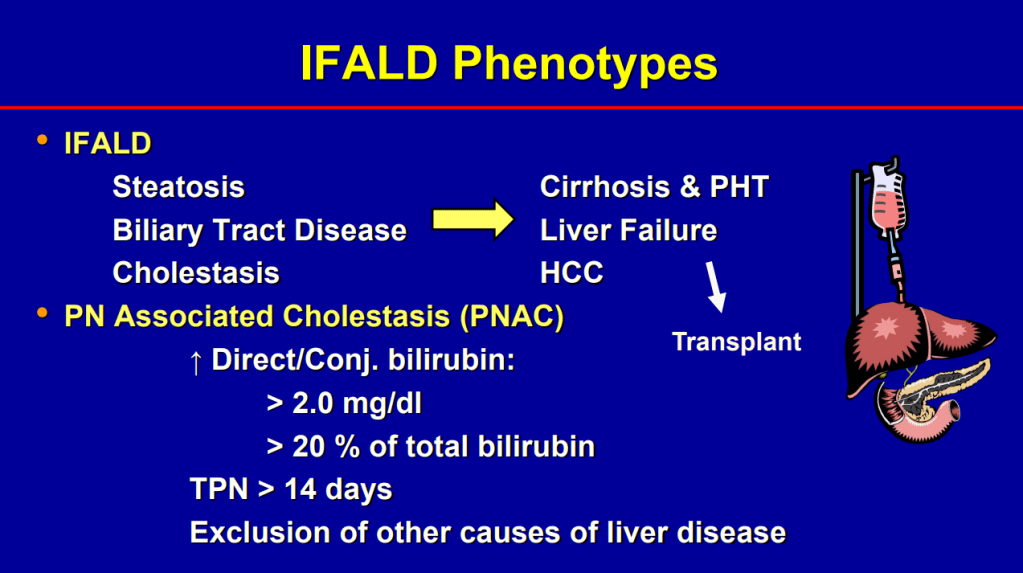
- Biliary cirrhosis related to parenteral nutrition has been the major indication for small bowel transplantation/multi-visceral transplantation. IFALD presentations: Steatosis, biliary tract disease and cholestasis
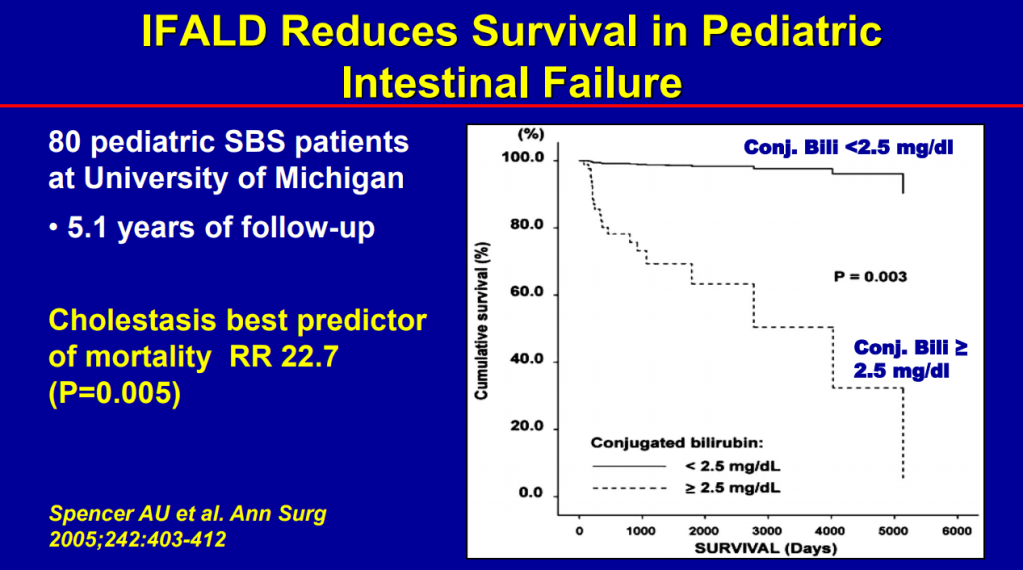
- Conjugated bilirubin >2.5 had RR 22.5 for mortality (prior to availability of intestinal transplantation)
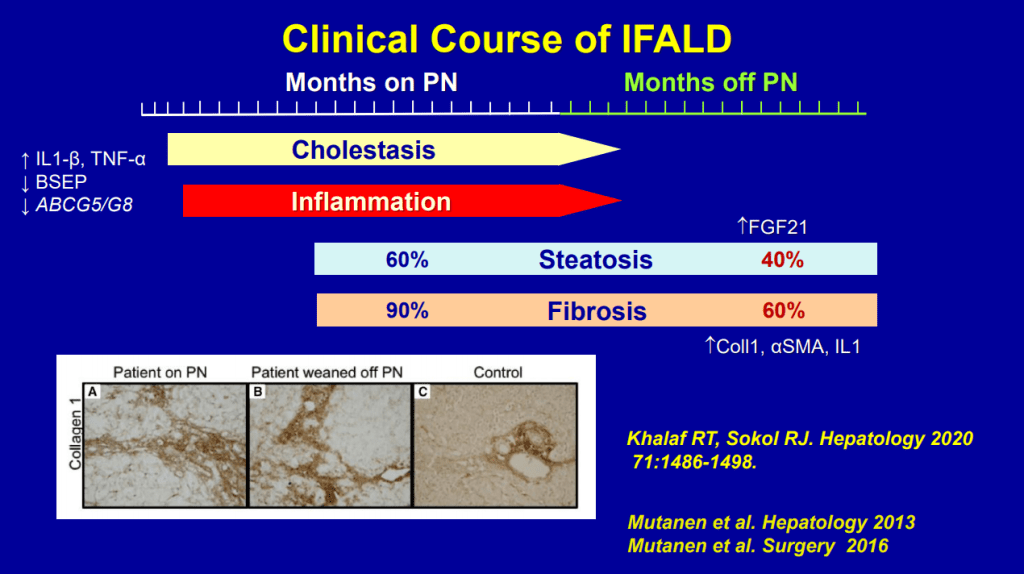
- Even after weaning off PN, studies have shown long-lasting fibrosis and steatosis in more than 40% of patients (>8 yrs off PN)
- Intestinal microbiome is altered in patients with IFALD
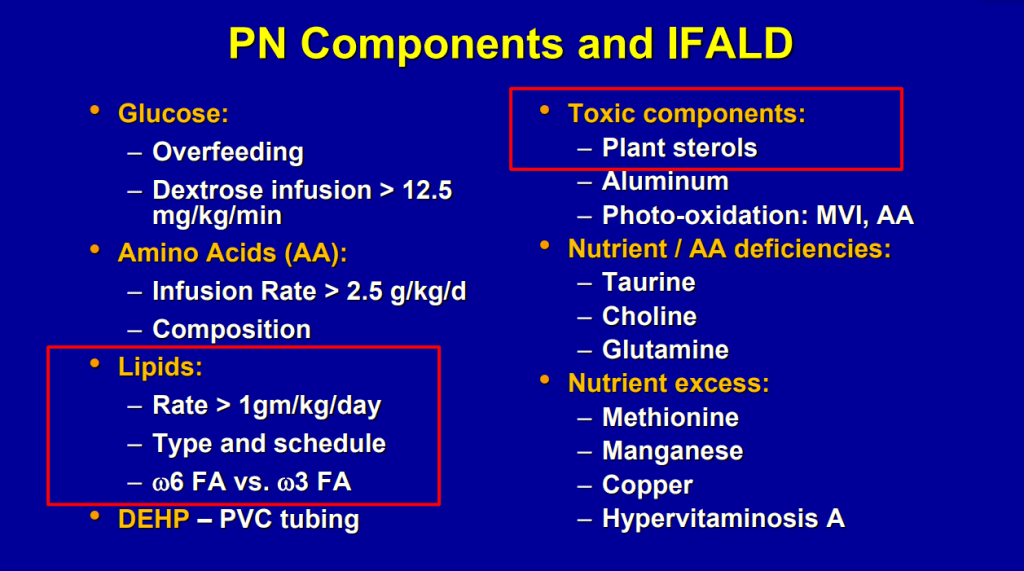
- Puder M et al. (Ann Surg 2009; 250: 395) showed that fish oil (at lower doses) was associated with improvement/resolution of parenteral nutrition associated cholestasis (PNAC)
- Lipid reduction also is associated with cholestasis resolution
- Treatments: Advance enteral feeds, lipid modulation, prevent CLABSI, treat bacterial overgrowth, GLP-2, and STEP procedure/tapering
- SMOF lipid allows full dosing of lipids (3 gm/kg)
- Caution with Fish oil (omegaven): 1. Does not prevent hepatic fibrosis progression 2. Reduction of lipid doses can have negative effects on brain growth
- Lipid management has been crucial in reducing the number of children needing intestinal transplantation
Some of the slides:




IBAT Inhibitors Frederick Suchy
Key points:
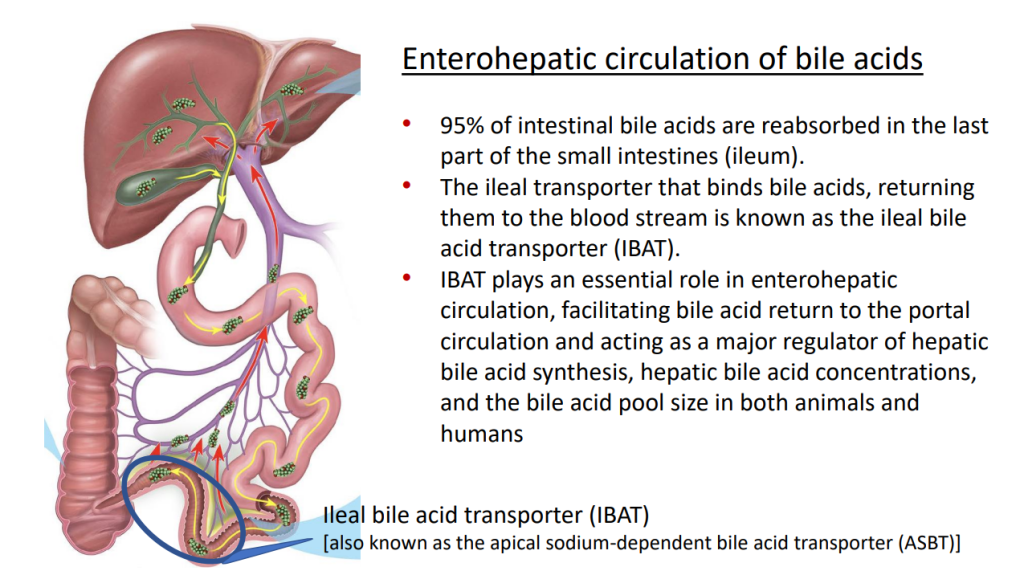
- IBAT inhibitors block intestinal absorption of bile acids/disrupt enterohepatic circulation; this leads to augmented bile acid excretion in stools
- IBAT inhibitors may reduce liver damage in the setting of cholestasis/accumulation of toxic bile acids
- Potential diseases for IBAT inhibitors include Alagille syndrome and PFIC
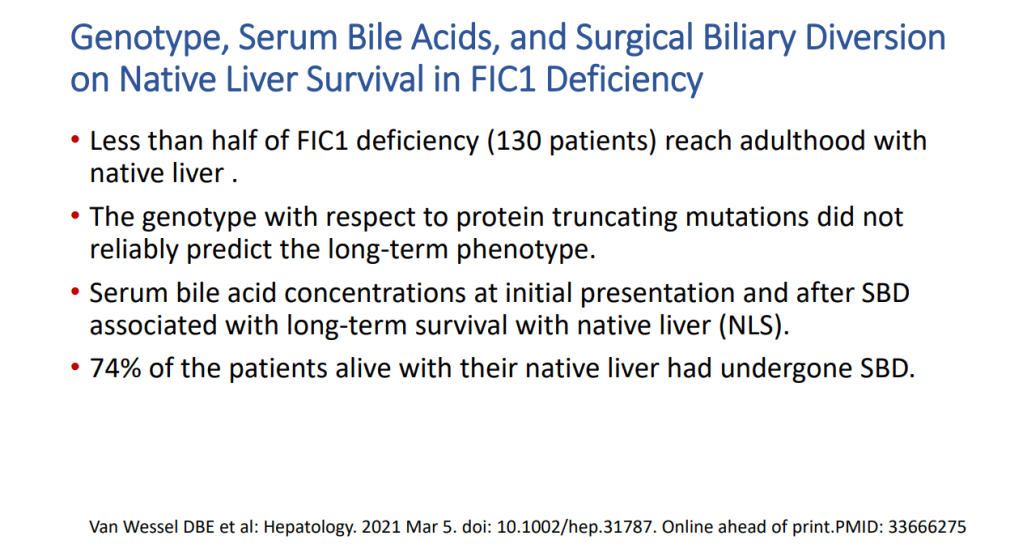
- Van Wessel et al (J Hepatol 2020; 73: 84-93) correlated survival with PFIC1/PFIC2 with bile acid levels and showed improvement in survival in those with surgical biliary diversion
- Goals for IBAT inhibitor trials: improvement in pruritus, bile acids, reduced ALT, hepatic fibrosis, HCC and need for liver transplantation
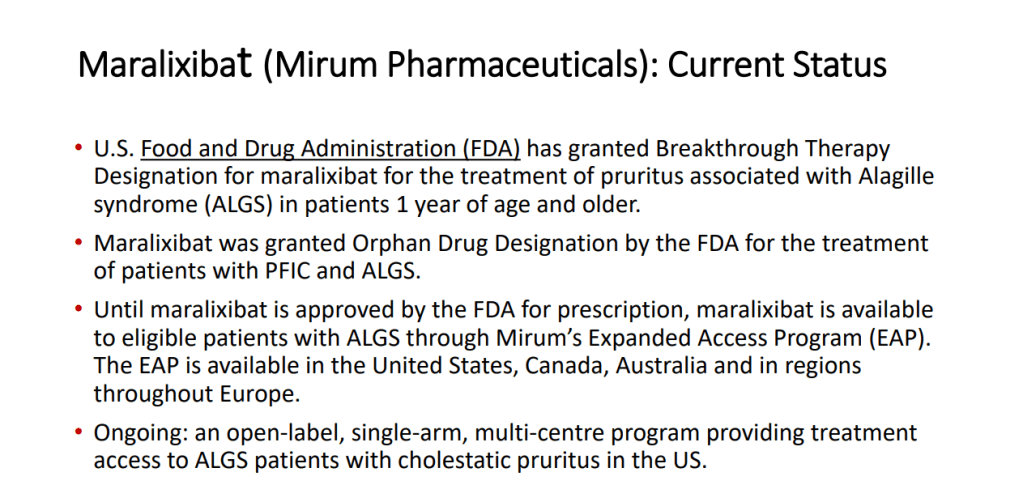
- Marixibat is available for use as an FDA approved breakthrough medication for Alagille and PFIC2 in pediatric patients older than 1 year
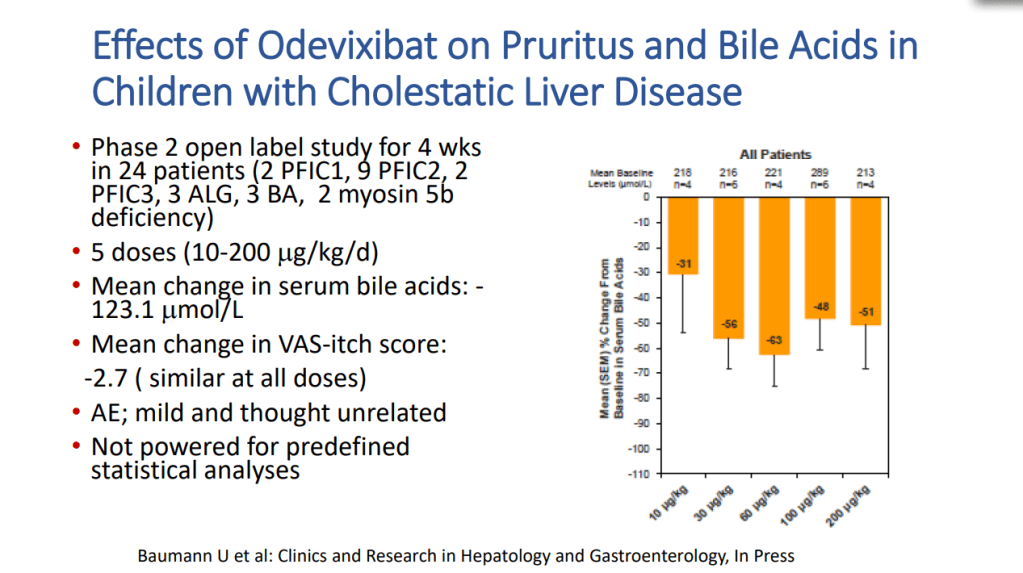
- Odexibat is designated as an orphan drug for Alagille, PFIC, PBC, and biliary atresia
- Safety appears good with IBAT inhibitors. Fat soluble vitamin monitoring is needed





Case report: Alejandro Velez Lopez
3 yo presented with fatigue and jaundice, 3 weeks after COVID-19 infection. She was not taking any medications. Labs: ALT 939, AST 1321, T bili 5.5, D bili 0.9, INR 2, Plts 174, Hgb 12.8, LDH 1297. remained positive for SARS-CoV2 by PCR. Acetaminophen -no exposure. Evaluation: LKM 1:1280. Neg ANA, NL Ferritin, NL sIL2r, Other viral studies negative, NL IgG. Developed encephalopathy with NH4 317, INR peaked at 2.8. Treated with steroids, rifaximin and lactulose. Liver biopsy showed sub-massive necrosis and fibrosis (indicative of autoimmune hepatitis, likely triggered or exacerbated by COVID-19). Patient responded to medical therapy and did not require liver transplantation.


