SA Harrison et al. NEJM 2024; 390: 497-509. A Phase 3, Randomized, Controlled Trial of Resmetirom in NASH with Liver Fibrosis
This “MAESTRO-NASH” study enrolled 966 adult patients biopsy-confirmed NASH (now termed MASH) and a fibrosis stage of F1B, F2, or F3. Approximately 60% of each arm had F3 fibrosis. Patients were randomly assigned in a 1:1:1 ratio to receive once-daily d resmetirom at a dose of 80 mg or 100 mg or placebo; Resmetirom is an oral, liver-directed, thyroid hormone receptor beta–selective agonist.
Key findings:
- MASH “resolution with no worsening of fibrosis was achieved in 25.9% of the patients in the 80-mg resmetirom group and 29.9% of those in the 100-mg resmetirom group, as compared with 9.7% of those in the placebo group (P<0.001)”
- “Fibrosis improvement by at least one stage with no worsening of the NAFLD activity score was achieved in 24.2% of the patients in the 80-mg resmetirom group and 25.9% of those in the 100-mg resmetirom group, as compared with 14.2% of those in the placebo group (P<0.001)”
- “Levels of a broad range of atherogenic lipids and lipoproteins, including LDL cholesterol, non-HDL cholesterol, triglycerides, apolipoprotein B, and lipoprotein(a), appeared to be reduced by resmetirom relative to placebo, findings consistent with those of earlier studies.18,19“
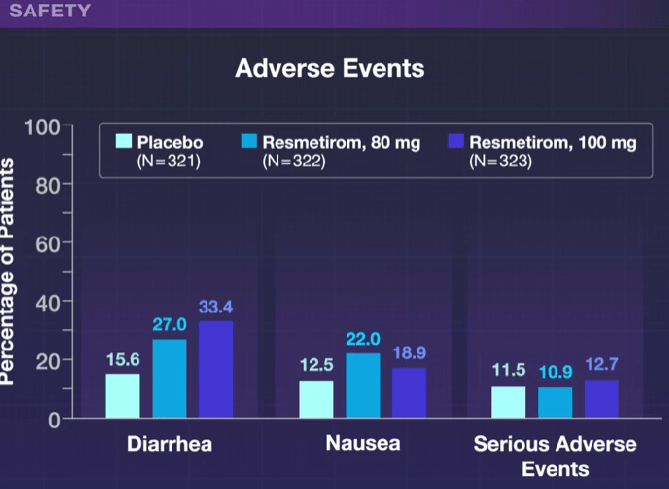
- Diarrhea and nausea were more frequent in the resmetirom group compared to placebo, though there were no differences in serious adverse effects. Patients in the 100 mg group were more likely to discontinue treatment (~7%) compared to 2% in the other two groups.
- “In this trial, achievement of a 30% reduction in hepatic fat (MRI-PDFF) or a 120% increase in the sex hormone–binding globulin level appeared to be associated with biopsy responses.”
In their discussion, the authors note that “Noninvasive testing to identify patients with NASH for treatment and to monitor treatment response will be important in clinical practice in which liver biopsy is infrequently used.”
The associated editorial by Kenneth Cusi (pg 559-561) notes the following:
- Resmetirom had neutral effects on body weight and insulin resistance.
- “Treatment affected the pituitary–thyroid hormone axis, with prohormone free T4 levels decreasing by approximately 17 to 21% and mean thyrotropin levels also decreasing.” It is unclear if this has any long-term significance (long-term data needed). ”Careful surveillance to detect early endocrine disease that is related to potential thyroid, gonadal, or bone disease appears warranted to avoid any potential risks from long-term treatment.”
- When subtracting the placebo effect, he notes that “approximately 2 of 10 patients treated will have NASH resolution and approximately 1 of 10 patients treated will have fibrosis improvement.” Thus, combination therapy may be needed.
My take: This study brings us a step closer to having a medication which can improve MASH as currently there are no FDA-approved medications. My speculation is that medications which achieve persistent weight loss will have a more pronounced effect on liver health and overall health.


Related blog posts:






