Dani Blum, NY Times 8/18/25: A Common Weight Loss Drug Can Treat Severe Liver Disease, F.D.A. Says
An excerpt:
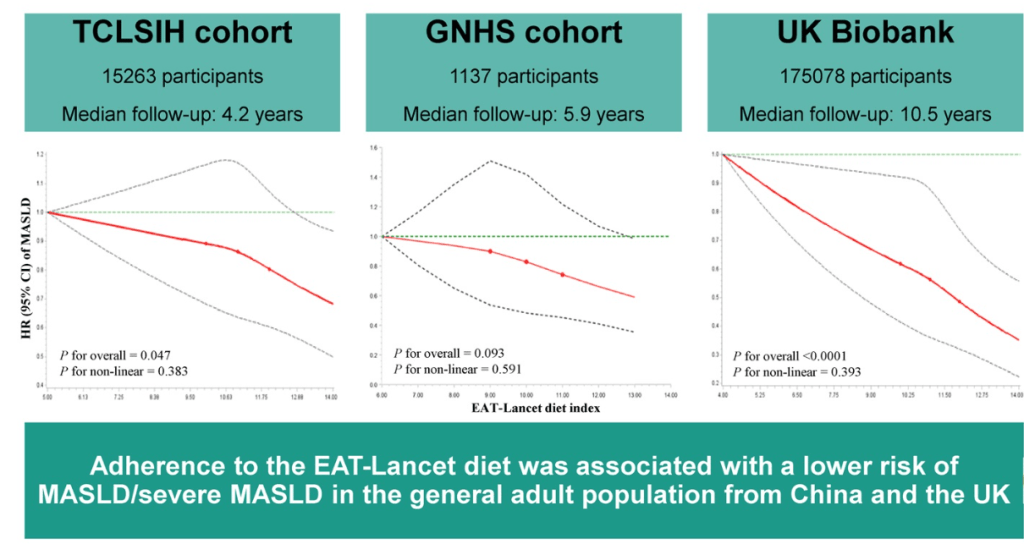
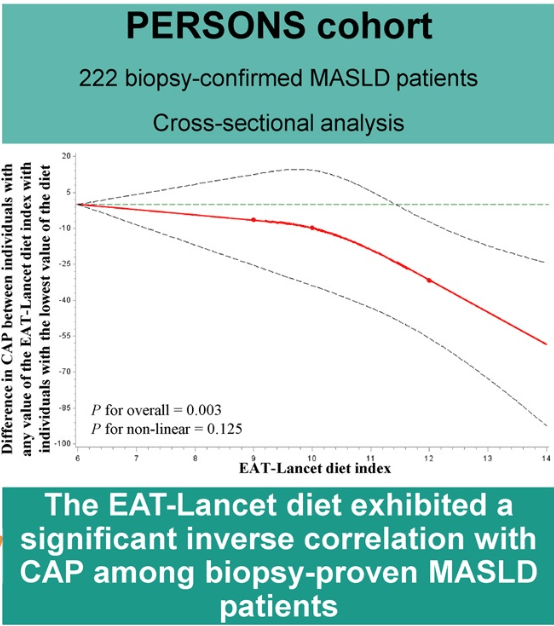
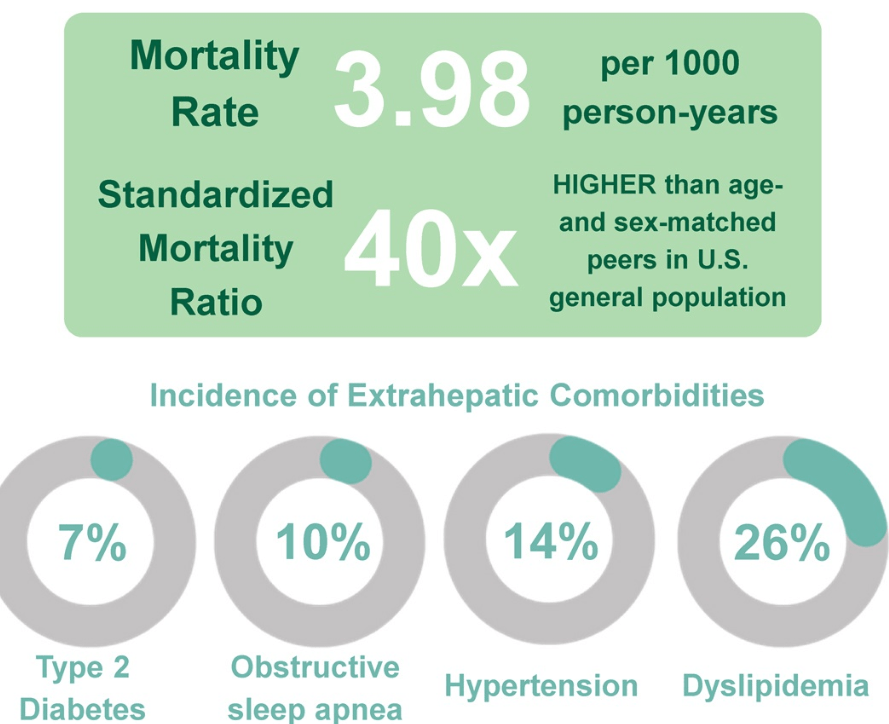
Roughly 15 million people — six percent of adults in the United States — have metabolic dysfunction-associated steatohepatitis, known as MASH. Rates of the disease are rising…
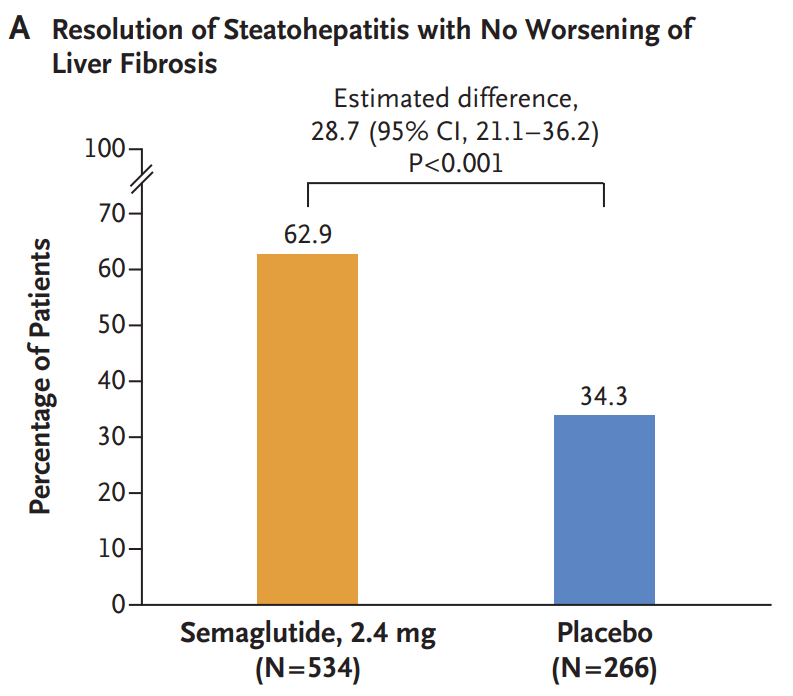
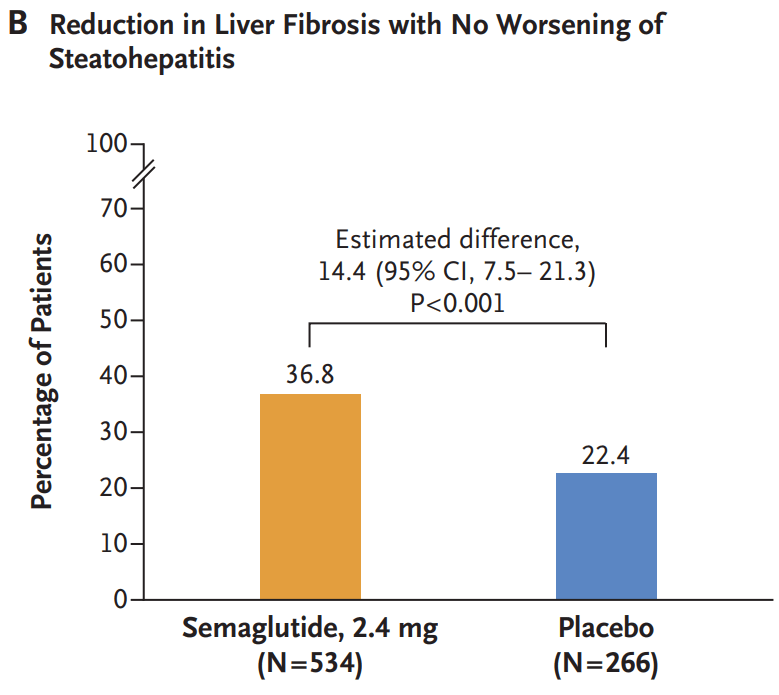
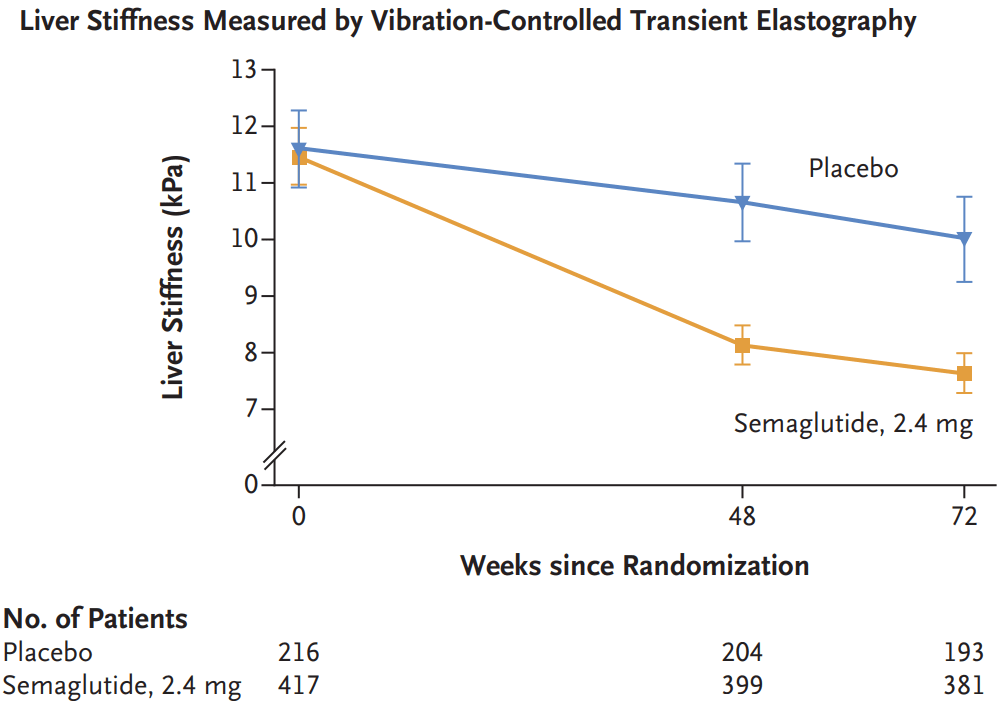
Wegovy, which is a weekly injection, is now approved for adults with MASH and moderate-to-advanced levels of fibrosis, or excessive scar tissue in the liver. The drug is not intended for people with cirrhosis…
Wegovy will be a welcome addition to the options doctors can prescribe — as long as their patients can access them. The drug carries a list price of over $1,300 a month, although most people do not pay that full amount. Many people have lost insurance coverage for weight-loss drugs, as plans struggle to keep up with the costs.
Related review article: G Targher et al. NEJM 2025; 393: 683-698. Metabolic Dysfunction–Associated Steatotic Liver Disease. This review article succinctly covers the epidemiology, manifestations, disease progression and pivotal pharmacologic advances.
Related blog post: Semaglutide’s Efficacy in Phase 3 MASH Trial