Yesterday’s pumpkin -please no snide remarks about how I can now retire and become a sculptor:

BE Sands et al. N Engl J Med 2024;391:1119-1129. Phase 2 Trial of Anti-TL1A Monoclonal Antibody Tulisokibart for Ulcerative Colitis
Background: “Several studies have implicated human tumor necrosis factor–like cytokine 1A (TL1A) in the pathogenesis of inflammatory bowel disease…Tulisokibart (formerly PRA023) is a humanized IgG1 kappa monoclonal antibody that binds to the membrane-bound and soluble forms of TL1A with high affinity and specificity. Tulisokibart prevents the interaction of TL1A and DR3, thereby suppressing type 1 and type 17 helper T-cell responses, increasing regulatory T-cell activity, and decreasing profibrotic pathways.”
Methods: (ARTEMIS-UC trial) The authors “randomly assigned patients with glucocorticoid dependence or failure of conventional or advanced therapies for ulcerative colitis to receive intravenous tulisokibart (1000 mg on day 1 and 500 mg at weeks 2, 6, and 10) or placebo. Cohort 1 included patients regardless of status with respect to the test for likelihood of response. Cohort 2 included only patients with a positive test for likelihood of response.”
“The inclusion of an integrated assessment of a panel of genetic markers as a diagnostic assay was based on the notion that patients with a propensity to overexpress TL1A might be more likely to have a response to tulisokibart than an unselected population.”
Key findings:
- In the first cohort, a significantly higher percentage of patients who received tulisokibart had clinical remission than those who received placebo (26% vs. 1%), endoscopic healing (31% vs. 4%), endoscopic improvement (37% vs 6%) and clinical response (66% vs 22%)
- “Among patients with a positive test for likelihood of response (cohorts 1 and 2 combined), clinical remission occurred in a higher percentage of patients who received tulisokibart than in those who received placebo (32% vs. 11%).”
- Improvement in CRP and Calprotectin were noted as early as 2 weeks and 6 weeks respectively
- The incidence of adverse events was similar in the tulisokibart and placebo groups
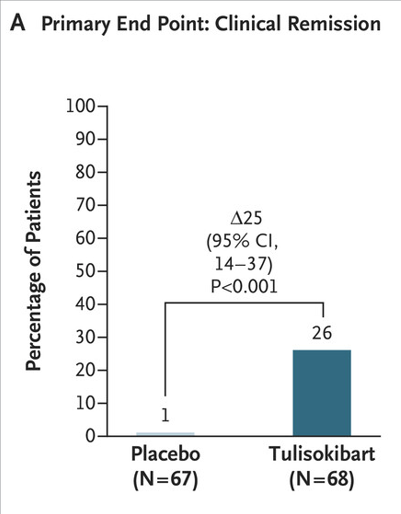

My take: Tulisokibart was effective in a group of patients with moderately to severely active ulcerative colitis who were refractory to advanced therapies.
Related blog posts:
- Dr. Joel Rosh: Positioning Therapies for Pediatric Ulcerative Colitis
- Risankizumab for Ulcerative Colitis
- Does Accelerated Dosing of Infliximab Work for Acute Severe Ulcerative Colitis?
- ENTERPRET: Vedolizumab Optimization Study in Ulcerative Colitis
- Upadacitinib vs Ustekinumab for Ulcerative Colitis