AL Lightner et al. Inflamm Bowel Dis 2023; 29: 1912-1919. A Phase I Study of Ex Vivo Expanded Allogeneic Bone Marrow–Derived Mesenchymal Stem Cells for the Treatment of Pediatric Perianal Fistulizing Crohn’s Disease
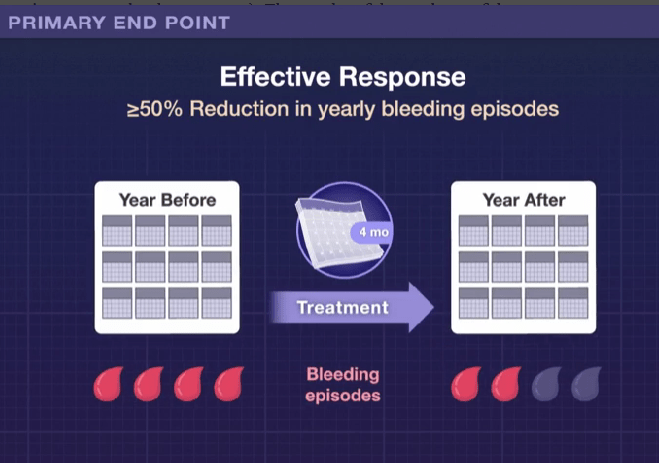
Seven pediatric patients with perianal Crohn’s disease were treated with mesenchymal stem cells. Key finding: At 6 months, 83% had complete clinical and radiographic healing. This healing rate is higher than “the 50% efficacy reported by the only completed randomized control phase III clinical trial.[ADMIRE study].”
MA Baarslag et al. NEJM 2023; 389: 1790-1796. Severe Immune-Related Enteritis after In Utero Exposure to Pembrolizumab
This case report details severe immune-related gastroenterocolitis after in utero exposure to pembrolizumab, an anti–PD-1 agent; the infant presented at 4 months of life. Extensive testing did not identify any underlying causes of VEO-IBD. This infant required TPN for a short period, but subsequently responded to treatment with glucocorticosteroids and infliximab (with plans to continue until at least 3 years of age). Both programmed death 1 (PD-1) and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) immune checkpoint inhibitors are negative regulators of T-cell immune function. Inhibition of these targets, resulting in increased activation of the immune system and can result in medication-induced colitis in the patients who take them and potentially in infants exposed to these agents in utero.
M Bramuzzo et al. Inflamm Bowel Dis 2024; 30: 20-28. https://doi.org/10.1093/ibd/izad018. Efficacy and Tolerance of Thalidomide in Patients With Very Early Onset Inflammatory Bowel Disease
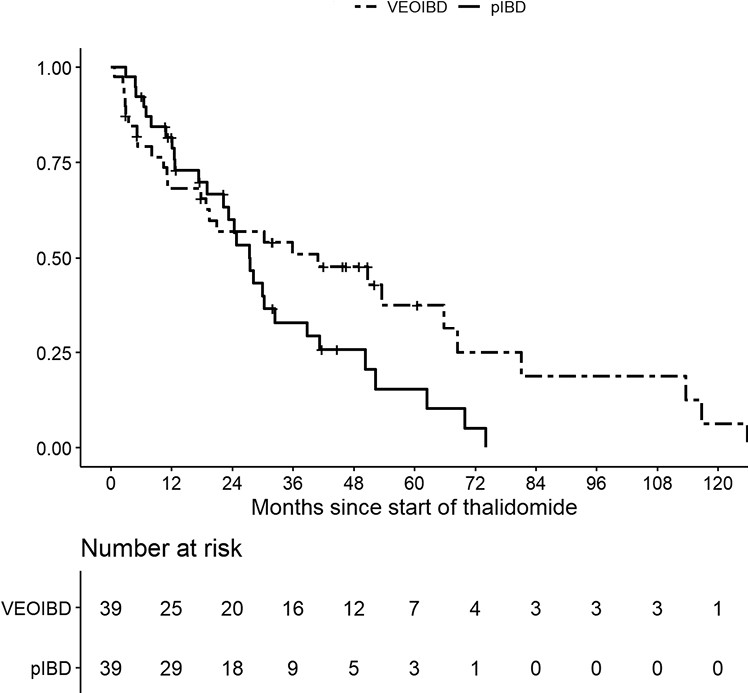
This retrospective study with 39 patients with VEO and 39 patients with pediatric IBD.
Key findings:
- The treatment persistence at 1, 2, and 3 years was 68.2%, 57.0%, and 50.9% for VEOIBD patients and 81.7%, 60.0% and 33.0% for pIBD patients, respectively
- A significantly higher proportion of VEOIBD patients discontinued therapy due to lack of efficacy (48.2% vs 17.2%; P = .03), while AEs were the main reason for discontinuation in pIBD patients
- A significatively lower number of VEOIBD patients experienced AEs compared with pIBD patients (14 [35.9%] vs 30 [76.9%]; P = .0005).
Treatment persistence:
Related blog posts: