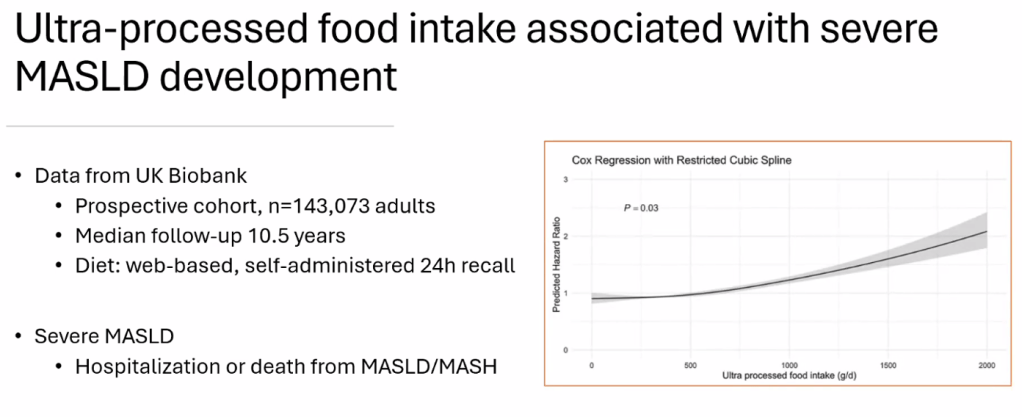
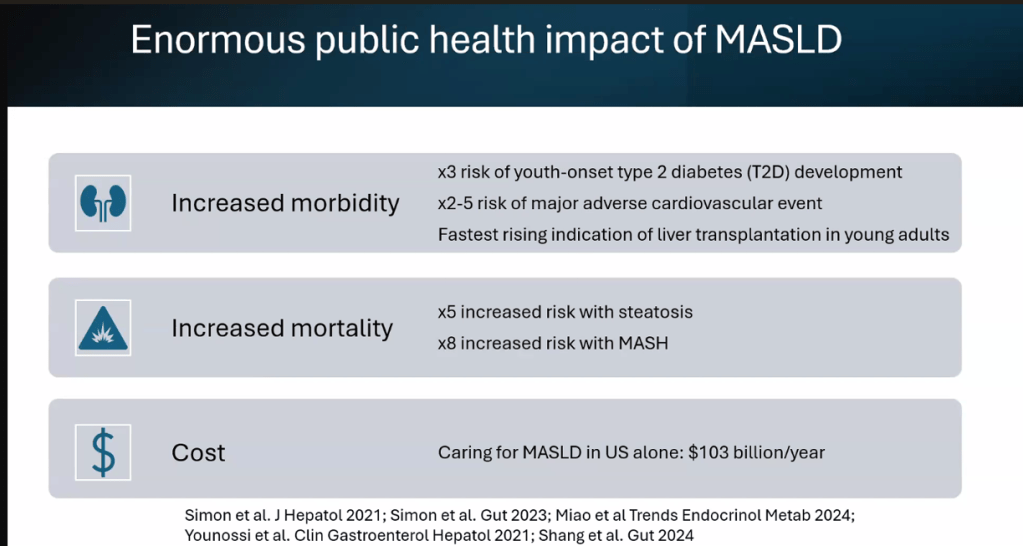
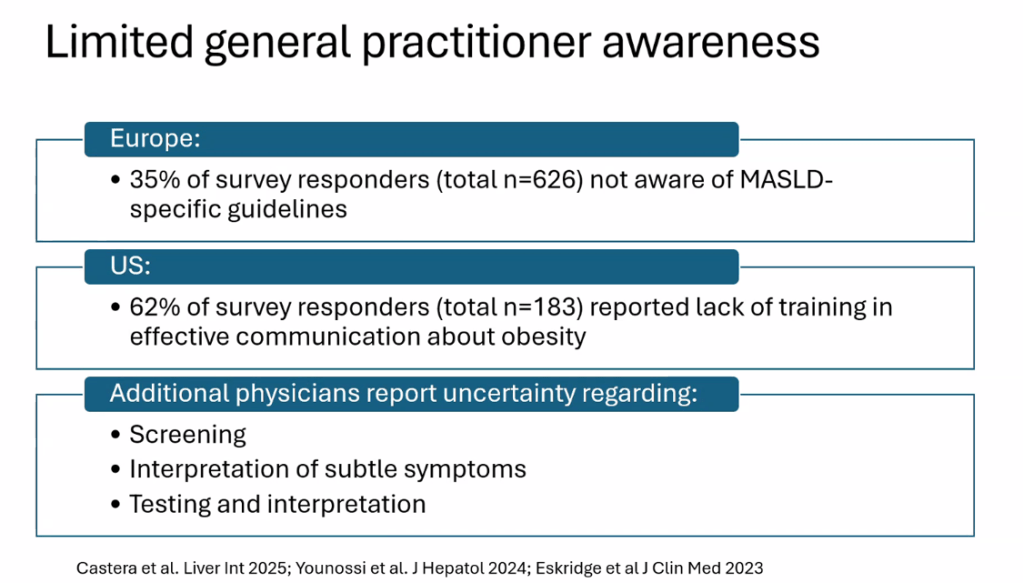
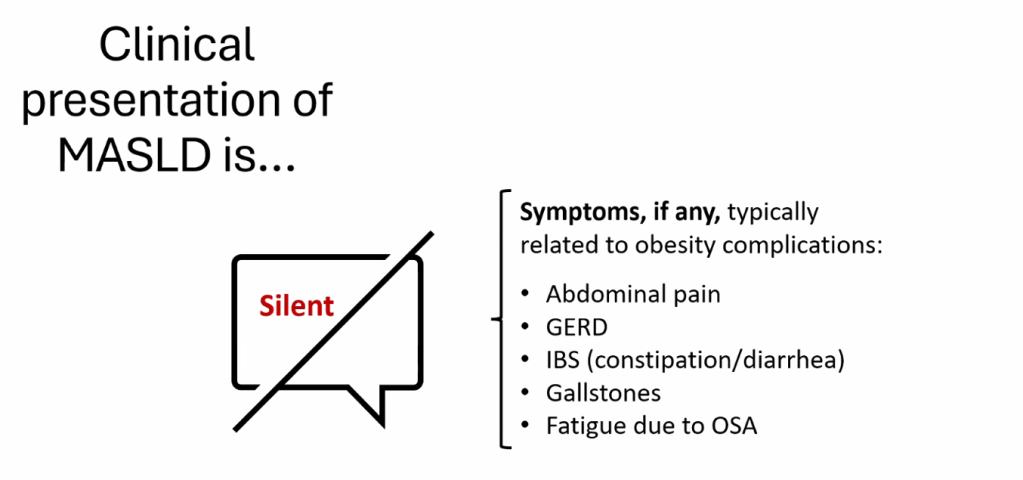
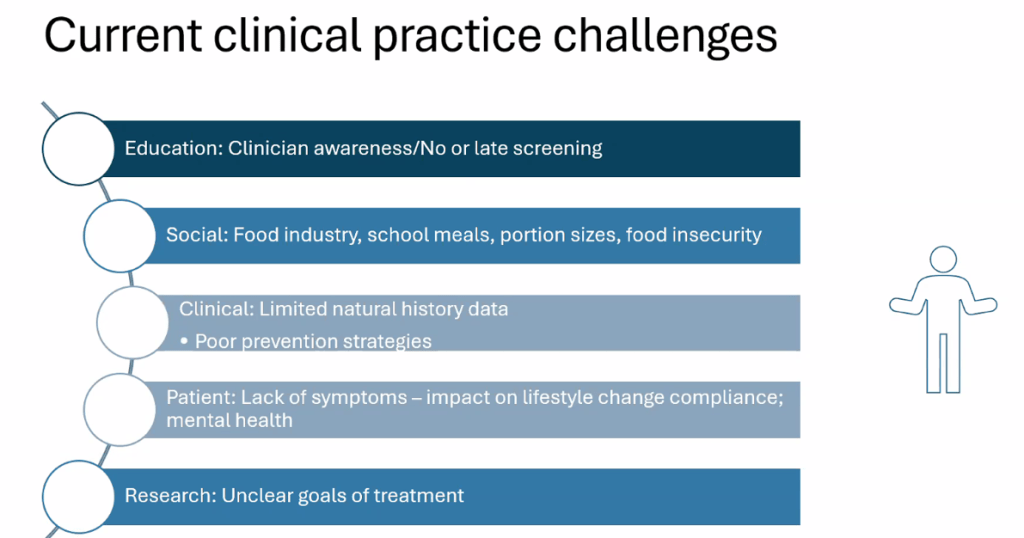
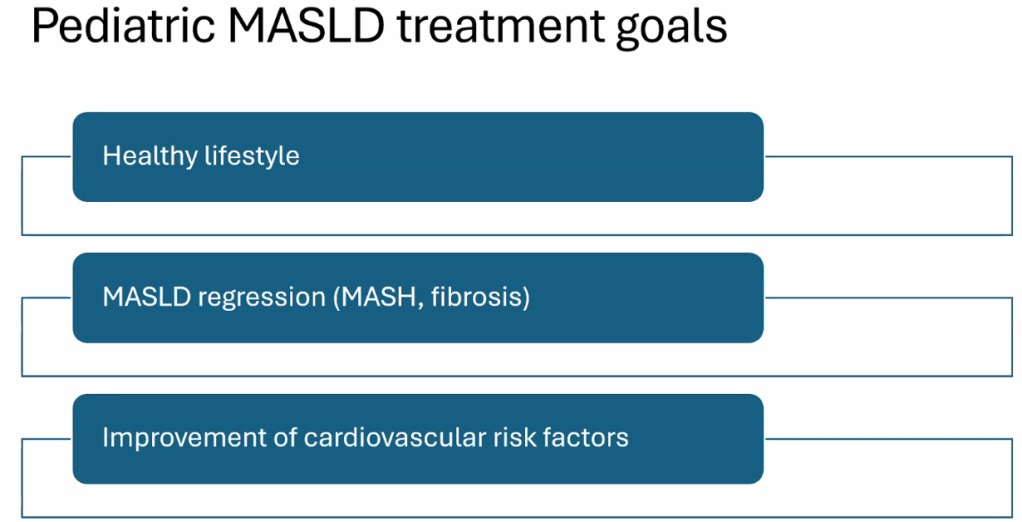
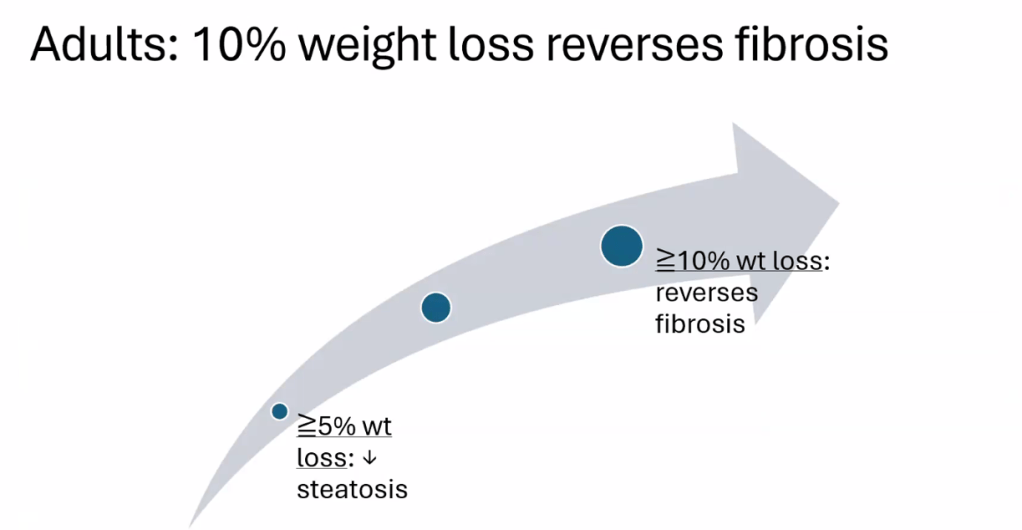
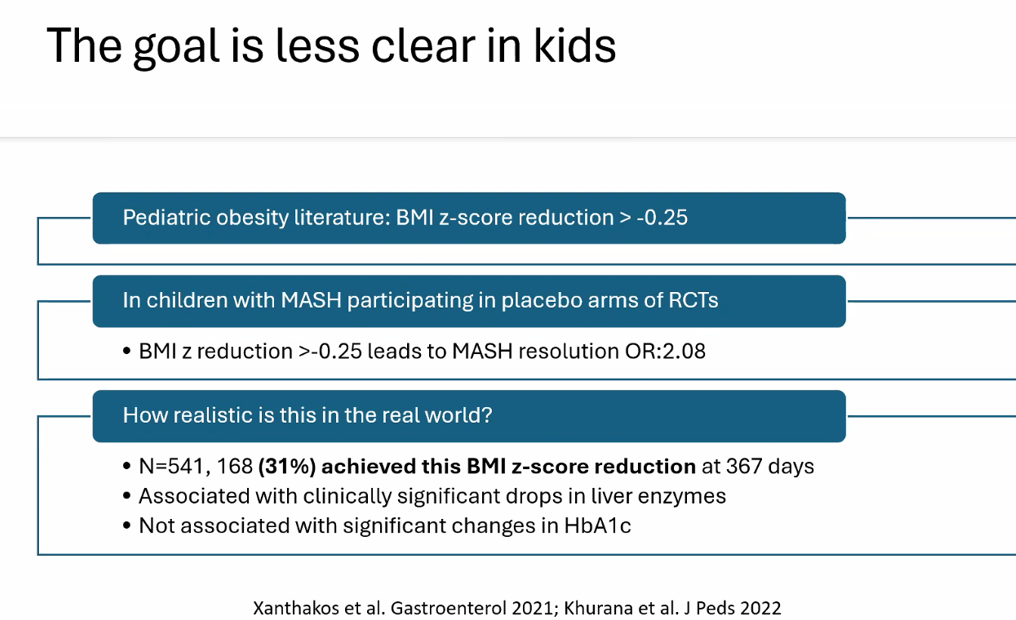
Happy July 4th!
L Gasoyan et al. Obesity 2025; DOI: 10.1002/oby.24331. Open Access! Changes in weight and glycemic control following obesity treatment with semaglutide or tirzepatide by discontinuation status
Methods: This retrospective cohort study used electronic health record data from a large health system in Ohio and Florida to identify adults with overweight or obesity without type 2 diabetes who initiated injectable semaglutide or tirzepatide between 2021 and 2023; 6109 received semaglutide, and 1772 received tirzepatide. Classification as high maintenance doses for semaglutide were 1.7, 2.0, or 2.4 mg and for tirzepatide 10.0, 12.5, or 15.0 mg, and all other dosages classified as low. The study grouped patients who discontinued pharmacotherapy into those who discontinued early (within 3 months of the index date) and late (within 3–12 months)
Key findings:
- 80.8% had low maintenance dosages
- Mean (SD) percentage weight reduction at 1 year was 8.7% (9.6%)
- ~50% discontinued medication within 1 year
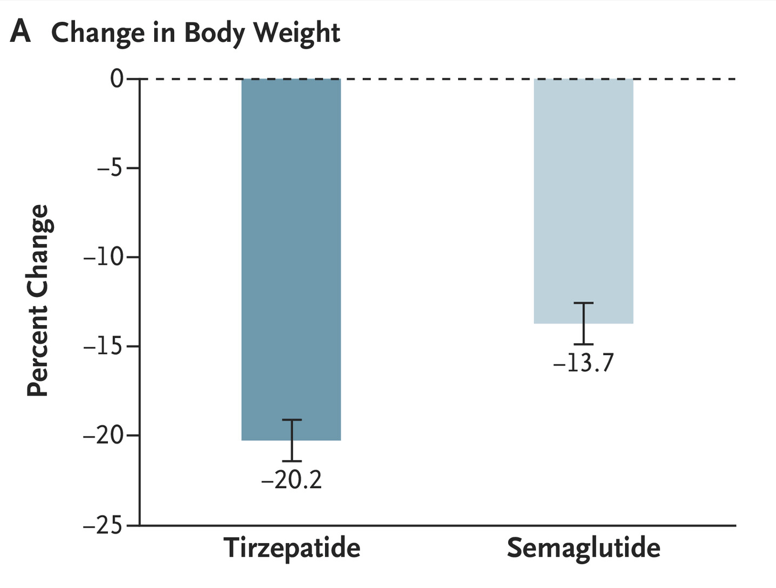
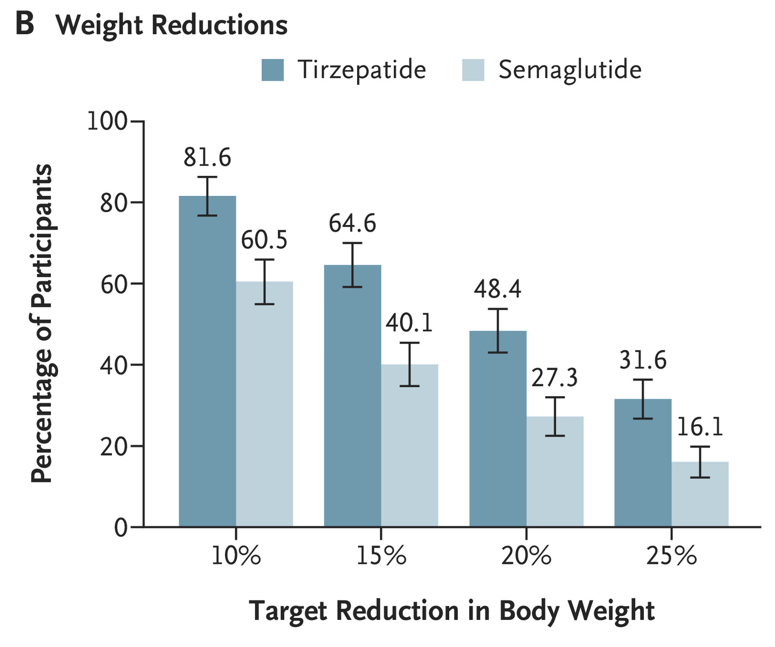
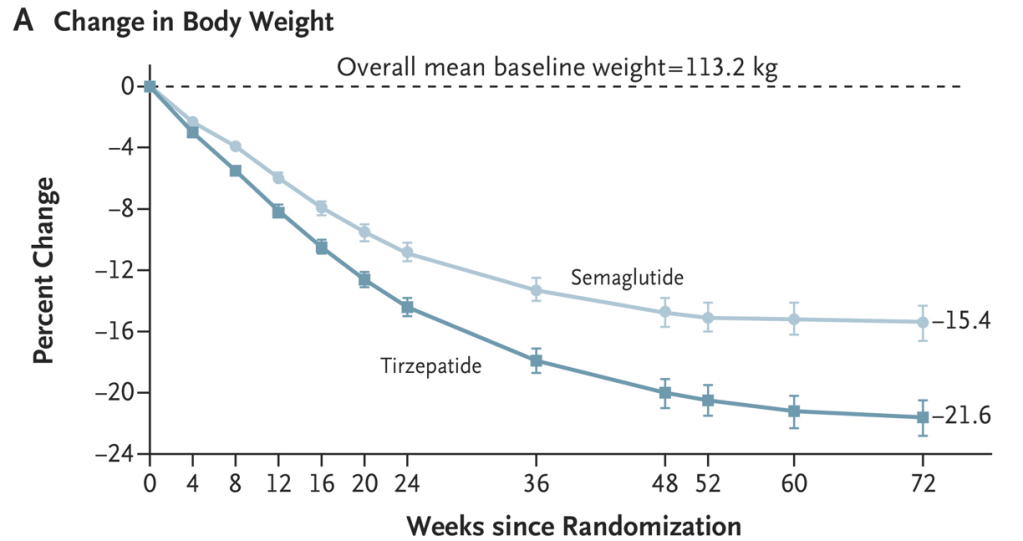
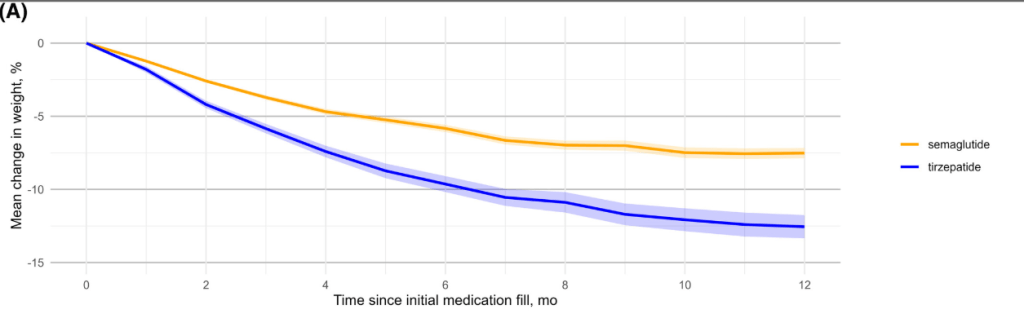
- Patients receiving tirzepatide had more weight loss than those receiving semaglutide (see below). Among patients who did not discontinue obesity pharmacotherapy at year 1, the mean (SD) percentage reduction in weight was 10.9% with semaglutide and 15.3% with tirzepatide
- In those receiving high dose medication, mean (SD) percentage reduction in weight was 14.7% with semaglutide and 18.0% with tirzepatide
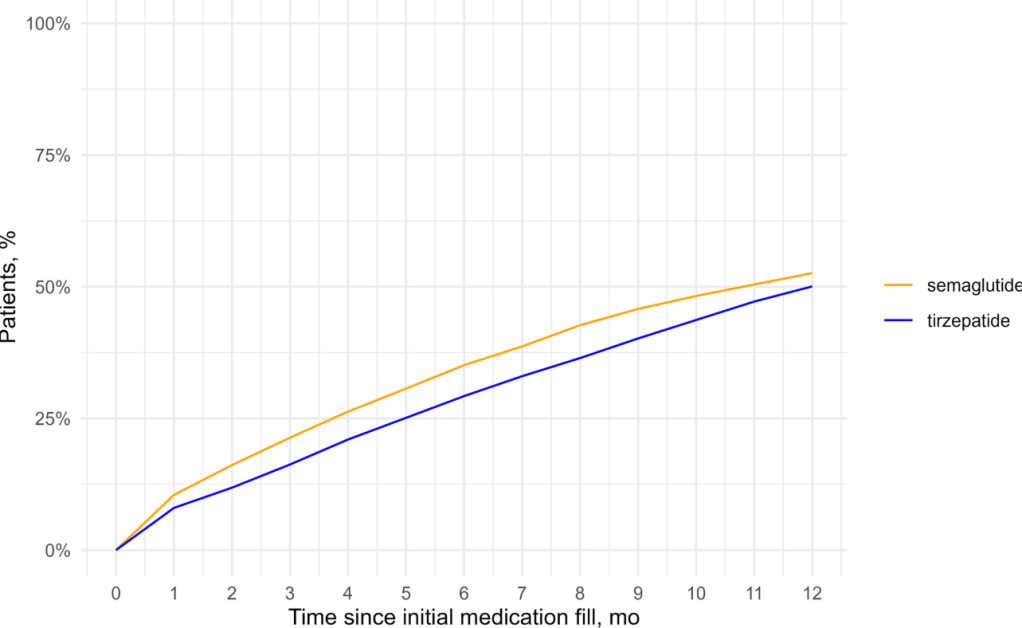
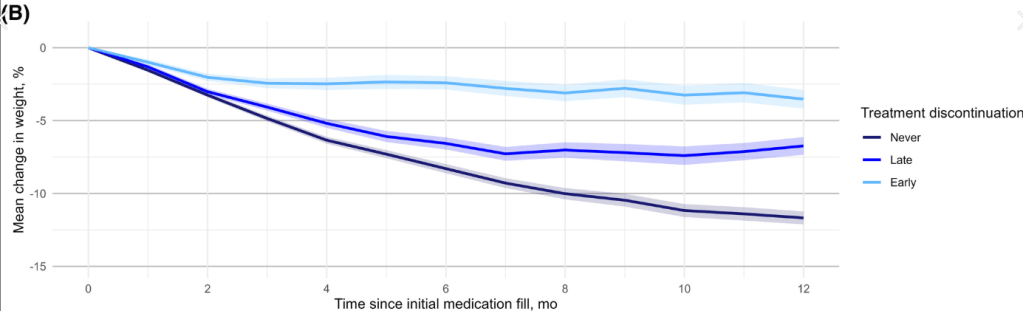
- Patients who continuing therapy had more weight loss than those who discontinued therapy (see below); Mean (SD) percentage weight reduction at 1 year was 3.6% (8.1%) with early discontinuation, 6.8% (9.1%) with late discontinuation, and 11.9% (9.2%) with non-discontinuation (p < 0.001).
DISCONTINUATION OF THERAPY:
SEMAGLUTIDE VS TIRZEPATIDE:
RESULTS WITH ONGOING TREATMENT VS TREATMENT DISCONTINUATION:
My take: This study showed higher rates of medication discontinuation in a real world setting compared to prior publications. In addition, the majority were receiving lower doses yet still achieving good results. However, increased discontinuation and lower doses likely explain the discrepancy in weight loss in this cohort which was less than in prior studies. It is important that patients taking these medications receive adequate counseling at the start to improve rates of adherence and long-term outcomes, including mitigation of muscle loss and bone loss.
Related blog posts:
- Head-to-Head: Tirzepatide Outperforms Semaglutide
- Lifetime Health Effects and Cost-Effectiveness of Tirzepatide and Semaglutide in US Adults
- Tirzepatide: Breakthrough in Obesity and Diabetes Management (SURMOUNT-1 Study at 3 years)
- Tirzepatide for Metabolic Dysfunction–Associated Steatohepatitis (MASH) & Uptick in GLP1 Use
- Key Insights on MASLD from Dr. Marialena Mouzaki
- Semaglutide Keeps Weight Off at Four Year Mark
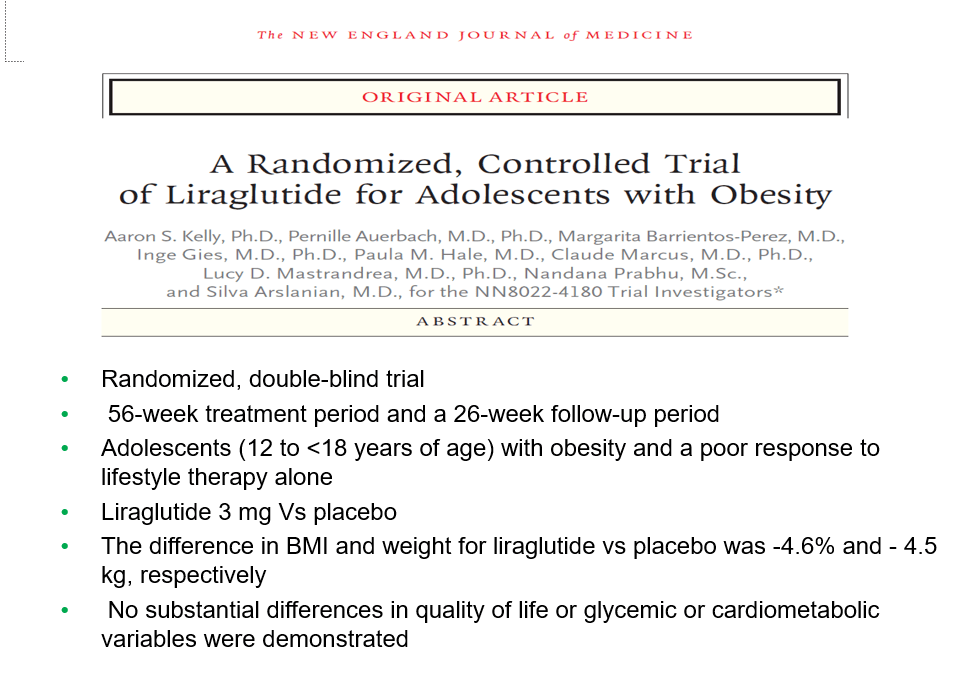
- Semaglutide in Adolescent Obesity
- Pharmacological Management of Pediatric Steatotic Liver Disease
- More Data Indicating GLP-1 Efficacy for MASH