The Good News:
MJ Buie et al. Inflamm Bowel Dis 2023; 29: 1536-1545. Open Access! Hospitalization Rates for Inflammatory Bowel Disease Are Decreasing Over Time: A Population-based Cohort Study
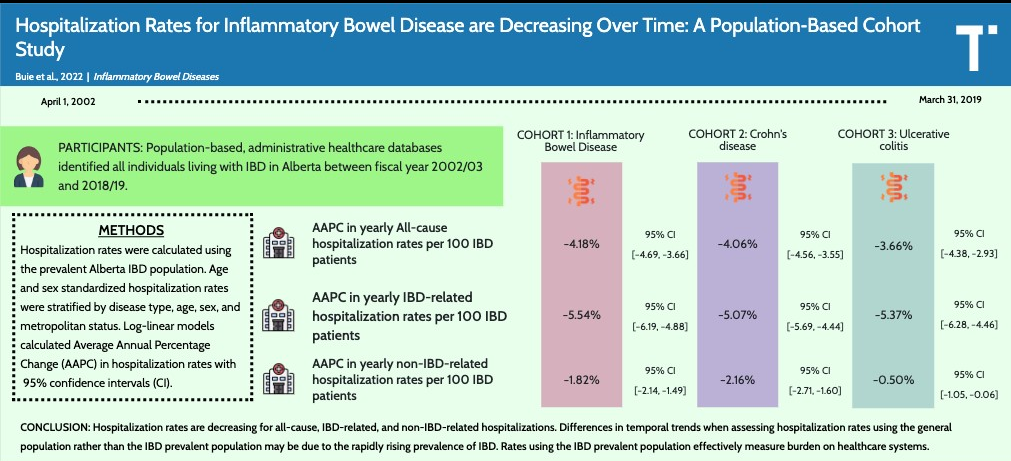
This population-based administrative data cohort study provides annual IBD hospitalization rates in Alberta, Canada.
Key findings:
- From 2002-2003 to 2018-2019, all-cause hospitalization rates decreased from 36.57 to 16.72 per 100 IBD patients (Average Annual Percentage Change (AAPC), −4.18%)
- Inflammatory bowel disease–related hospitalization rate decreased from 26.44 to 9.24 per 100 IBD patients (AAPC, −5.54%)
- The absolute number of hospitalizations, however, likely did not improve because this is affected by the increase in IBD prevalence. In Alberta, there was a 3-fold increase from 2002 to 2018 (general population increased 1.4 fold during this period)
“The last 2 decades have seen the introduction of several advanced therapies with novel mechanisms of action.22 The introduction of these therapies has been accompanied by changes in management strategies that include earlier introduction of advanced therapies based on risk stratification, treat-to-target, and monitoring strategies.5,23–26 These advancements include risk stratification, allowing for earlier introduction of advanced therapies; proactive clinical management algorithms to monitor disease activity; and therapeutic drug monitoring allowing for continued concentration-based dosing.23–26The net effect of these medical advances shifted IBD management from the hospital to the outpatient setting.27“
The Bad News:
DK Choi et al. Inflamm Bowel Dis 2023; 29: 1658-1661. Delays in Therapy Associated With Current Prior Authorization Process for the Treatment of Inflammatory Bowel Disease
This retrospective study of 1693 prior authorizations (PAs) from 2020-2021. Key findings:
- 1397 PA initially approved, 209 first-level PAs approved, 23 second-level PAs approve, and 11 external review requests approved. In total 97% (1640 of 1697) were approved
- Dose escalations had the lowest approval rate of 67.6%
- FDA approval had favorable OR for PA approval of 4.45
- The median time to biologic initiation was 21 days, with appeals causing further delays to initiation
Median Days to Determination by Insurance Level:
- Prior authorization: 11 days
- First level appeal: 29 days
- Second level appeal: 51 days
- External review request: 73 days
My take: The PA process usually results in few denials (if pursued) but does result in significant delays in therapy. At the same time, these newer therapies have been associated with improvement in hospitalizations rates.
Related blog posts:
- The Consequences of Prior Authorizations
- “Denials, Dilly-dallying and Despair”
- “We Need More Information to Process This Claim”
- For the Next Insurance Appeal: Therapeutic Drug Monitoring in Adalimumab Treatment (Pediatrics) & Satire on Prior Authorizations
- High Rates of Denying Medical Care for Medicaid Patients Managed by Health Insurers
- Kids Are Different: Therapeutic Drug Monitoring
- Do Anti-TNF Agents Reduce Surgeries and Hospitalizations?
- Proactive Therapeutic Drug Monitoring in Pediatric Crohn’s disease -Better Outcomes
