First about Ebola –here’s the Ebola recommendation from the NEJM editors regarding quarantine:
An excerpt:
The governors of a number of states, including New York and New Jersey, recently imposed 21-day quarantines on health care workers returning to the United States from regions of the world where they may have cared for patients with Ebola virus disease. We understand their motivation for this policy — to protect the citizens of their states from contracting this often-fatal illness. This approach, however, is not scientifically based, is unfair and unwise, and will impede essential efforts to stop these awful outbreaks of Ebola disease at their source, which is the only satisfactory goal…We should be honoring, not quarantining, health care workers who put their lives at risk not only to save people suffering from Ebola virus disease in West Africa but also to help achieve source control, bringing the world closer to stopping the spread of this killer epidemic.
Take-home message: Read the entire editorial why quarantine is not the right approach for asymptomatic returning health care workers.
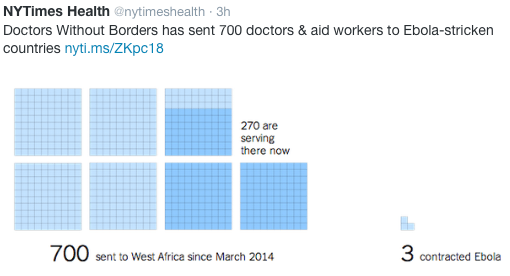
Direct Ebola Risk to Health Care Workers
Now in followup to yesterday’s post about HCV and diabetes:
Even Perry Mason would have had a difficult time proving hepatitis C virus (HCV) did not cause diabetes until a recent publication (Hepatology 2014; 60: 1139-49, editorial 1121-23).
In this study using population-based data from the U.S. National Health and Nutrition Examination Survey (NHANES) with 15,128 adult participants, the authors show that the prevalence of diabetes and prediabetes did not differ by HCV status. The authors used standardized definitions for diabetes and prediabetes and adjusted for major confounders. The authors did note a relationship between elevated alanine aminotransferase (ALT) with diabetes regardless of HCV status. In their cohort, 56.7% had normal glucose, 32.8% had prediabetes, 3.2% had undiagnosed diabetes, and 7.3% had diagnosed diabetes. The mean age progressively increased in these groups: 40.8 years, 51.9 years, 58.9 years, and 59.2 years respectively.
Among those with diabetes, 10.5% were HCV RNA-negative and 12.0% were HCV RNA-positive –unadjusted for ALT values; the unadjusted HCV antibody status was nearly identical at 10.5% and 10.2% respectively. After adjustment, the OR for being HCV RNA-positive was 1.06 (P=0.53) with confidence limits of 0.59-1.90.
In examining the evidence, the editorial and the discussion review previous evidence of a significant association between HCV infection, insulin resistance, and diabetes. The odds ratio for this association (HCV and diabetes) was estimated to be about 1.7. The problems with this association were the following:
- Much of the work was reported from tertiary care centers
- Advanced liver disease (of any type) is a well-established risk factor for type 2 diabetes (T2DM)
- Many studies may have included patients with nonalcoholic fatty liver disease which is another risk factor for diabetes
- These studies did not control for ALT values
Bottomline (from editorial): This study “calls one to reconsider the dogma on the role of IR [insulin resistance] in the pathogenesis of HCV infection and its association with T2DM.” If there is an association, it is much smaller than previous estimates.
Related blog post: Treating HCV Helps Diabetics | gutsandgrowth

