KR Mysore et al. J Pediatr Gastroenterol Nutr. 2025;80:549–558. Recent advances in the management of pediatric cholestatic liver diseases
This is a useful review summarizing advances in the management of cholestatic diseases.
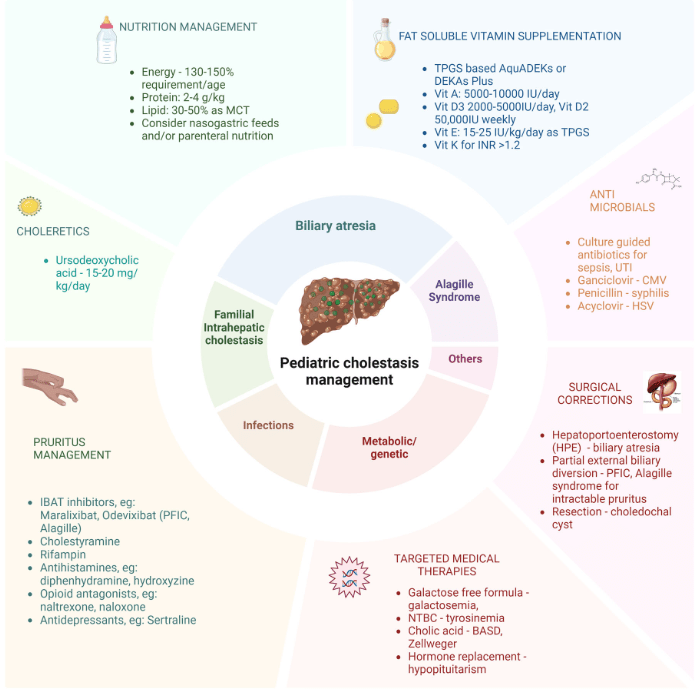
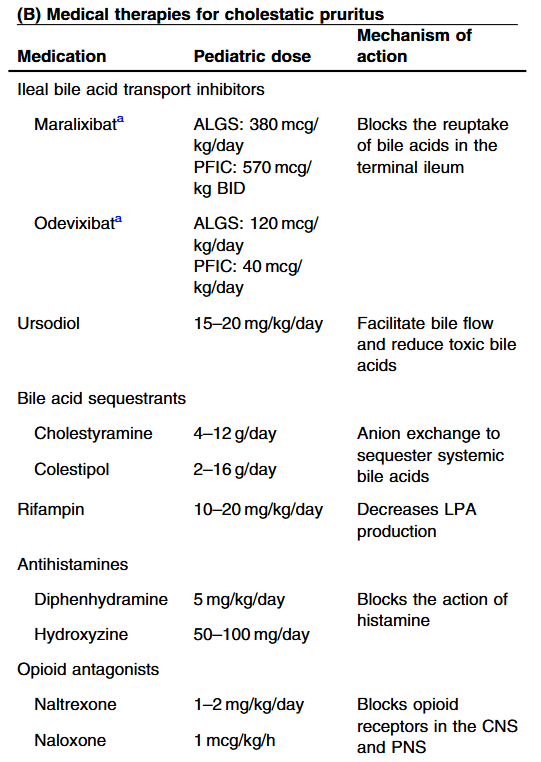
Treatment with IBAT inhibitors:
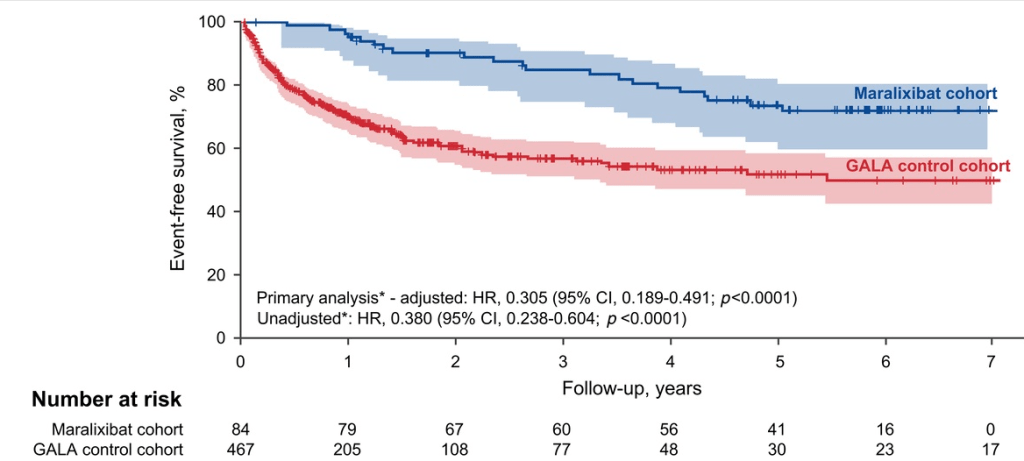
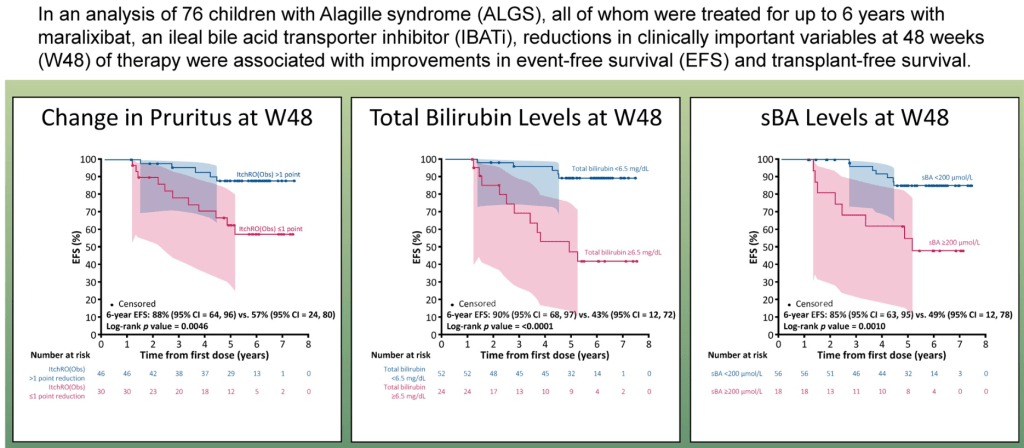
“Improvement in both pruritus and serum BAs/bilirubin levels has been associated with improved event‐free survival and 6‐year transplant‐free survival in ALGS patients treated with maralixibat. Additionally, this class of medication improved overall growth of the patient by improving mean height and weight Z scores that may be related to reduced impact of high serum bile acid levels on the growth axis although further studies are needed to better define the mechanism responsible for this out-come. This finding suggests these parameters could be used as surrogate end‐points for disease severity in diseases like ALGS or PFIC, where the time course to develop the need for LT commonly occurs over many years.”
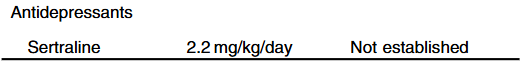
Related blog posts:
- Efficacy and Safety of Odevixibat with Alagille Syndrome (ASSERT Trial)
- Relooking at 6-Year Data of Maralixibat for Alagille Syndrome
- Lecture: IBAT Inhibitor for Alagille Syndrome
- GALA: Alagille Study
- START Study: Steroids Not Effective For Biliary Atresia (After Kasai)
- Liver Shorts: Malnutrition in Biliary Atresia, Cholestasis with ECMO, Impaired Cognition After Pediatric Liver Transplantation
- 30 -Year Outcomes with Biliary Atresia
Disclaimer: This blog, gutsandgrowth, assumes no responsibility for any use or operation of any method, product, instruction, concept or idea contained in the material herein or for any injury or damage to persons or property (whether products liability, negligence or otherwise) resulting from such use or operation. These blog posts are for educational purposes only. Specific dosing of medications (along with potential adverse effects) should be confirmed by prescribing physician. Because of rapid advances in the medical sciences, the gutsandgrowth blog cautions that independent verification should be made of diagnosis and drug dosages. The reader is solely responsible for the conduct of any suggested test or procedure. This content is not a substitute for medical advice, diagnosis or treatment provided by a qualified healthcare provider. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a condition.