KJ Moise et al. NEJM 2024; 391: 526: 526-537. Nipocalimab in Early-Onset Severe Hemolytic Disease of the Fetus and Newborn
Background: In early-onset severe hemolytic disease of the fetus and newborn (HDFN), transplacental transfer of maternal anti-erythrocyte IgG alloantibodies causes fetal anemia that leads to the use of high-risk intrauterine transfusions in order to avoid fetal hydrops and fetal death. Nipocalimab, an anti–neonatal Fc receptor (FcRn) blocker, inhibits transplacental IgG transfer and lowers maternal IgG levels.
Nipocalimab is under development for the treatment of multiple IgG autoantibody- or alloantibody-driven diseases. FcRn is the sole placental IgG transporter and salvage receptor that maintains circulating maternal serum IgG concentrations. FcRn blockade aims to inhibit alloantibody transfer to the fetus and to lower maternal IgG alloantibody titers
Methods: Phase II, open label study with weekly intravenous infusions of nipocalimab were administered to the maternal participants from baseline (~14 weeks gestation) until the planned last dose at 35 weeks’ gestation.
Key findings:
- Live birth at 32 weeks’ gestation or later without intrauterine transfusions occurred in 7 of 13 pregnancies (54%) in the study. No cases of fetal hydrops occurred, and 6 participants (46%) did not receive any antenatal or neonatal transfusions
- Live birth occurred in 12 pregnancies. The median gestational age at delivery was 36 weeks and 4 days
Discussion: “IVIG is used in some cases of early-onset severe HDFN on the basis, in part, of its competitive FcRn inhibition, which is similar to nipocalimab, but intrauterine transfusions are still used in the large majority of cases, despite the use of IVIG. The favorable outcomes that we observed with nipocalimab therapy are probably due to its FcRn-binding affinity, which is more than 1000 times that of IVIG and thus potentially affords greater inhibition of transplacental alloantibody transfer and lowering of the maternal alloantibody titer. The decrease in the maternal alloantibody titer of 4 to 32 times that was observed with nipocalimab, as compared with the decrease of 35 to 43% that was reported with IVIG.”
In the associated editorial (Maisonneuve et al. pg 563-567), the authors note that nipocalimab will “probably reduce the passive immunity of newborns…should follow surviving children. Nipocalimab treatment may also be useful for other fetal complications caused by transplacental transfer of maternal IgG, such as antiplatelet alloantibodies or for maternal autoimmune conditions caused by autoantibodies that cross the placenta and cause transitory autoimmune disease in the newborn, including …systemic lupus erythematosus with anti-SSA antibodies.”
My take: This therapy may also be an option in preventing subsequent cases of gestational alloimmune liver disease in at-risk mothers.
Related blog posts:
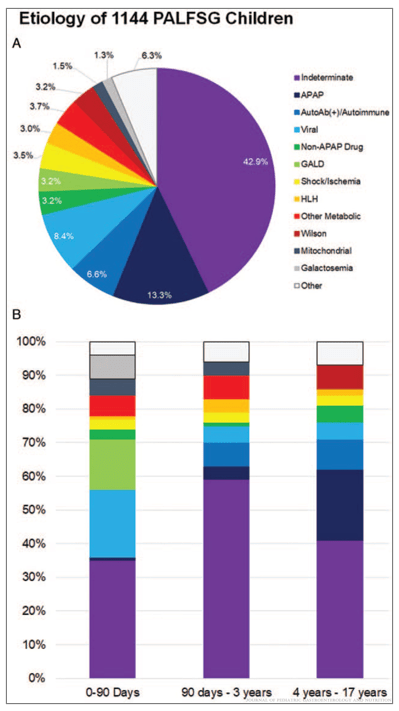
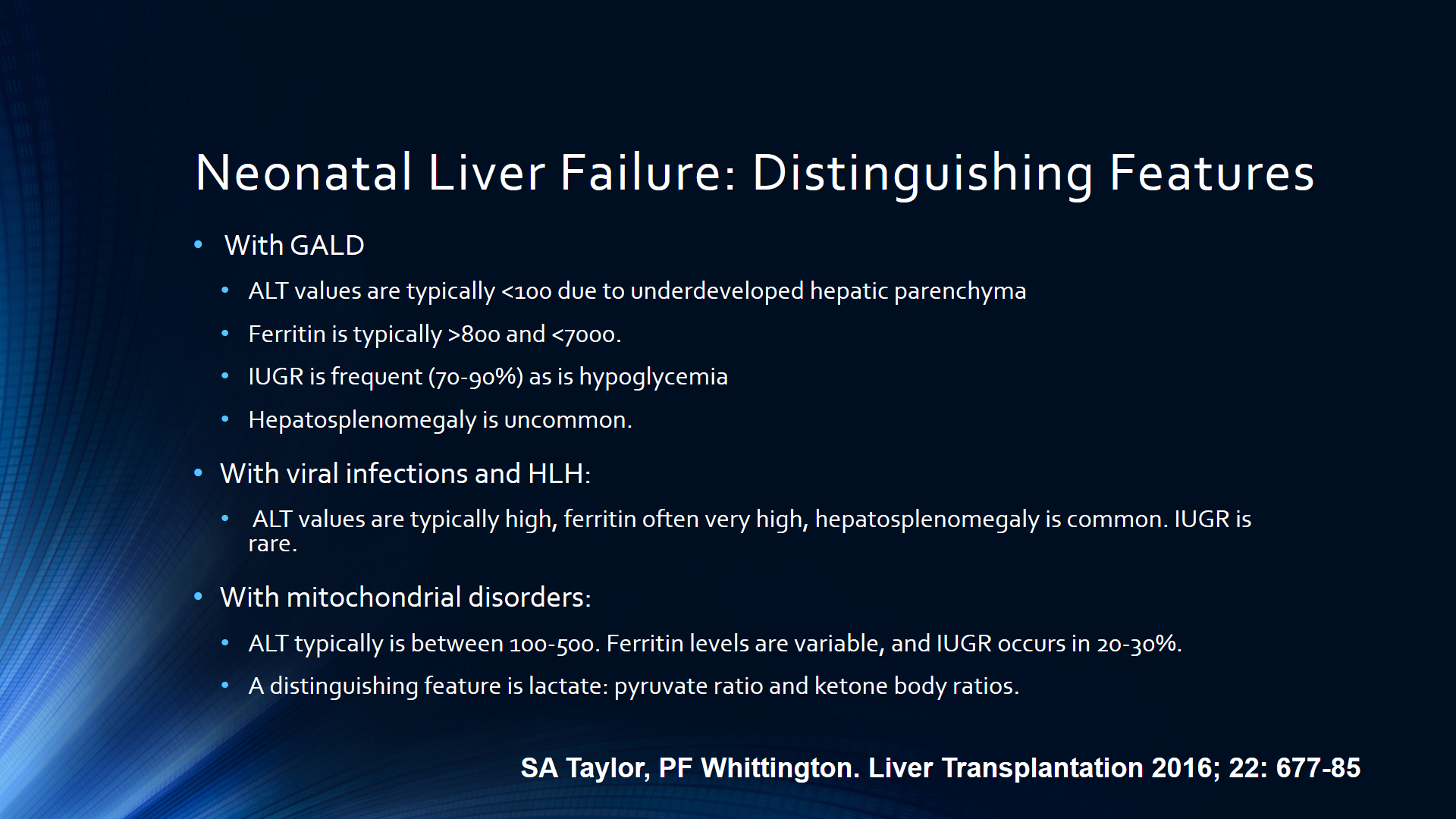
- Algorithm for Neonatal Acute Liver Failure
- Brief Updates: COVID-19/Hydroxychloroquine, GALD, Anorexia Nervosa, and Esophaeal-gastric Dissociation Outcomes
- A new GALD phenotype
- The more you know the more you see