C McDonald et al. Inflammatory Bowel Diseases, Volume 30, March 2024, Pages 423–428. Open Access! Higher Ustekinumab Levels in Maintenance Therapy are Associated with Greater Mucosal Healing and Mucosal Response in Crohn’s Disease: An Experience of 2 IBD Centers
This retrospective study enrolled 47 patients receiving maintenance ustekinumab (UST) for Crohn’s disease. Over one-third of patients (n = 18, 38.3%) were on higher than standard dosing of 90 mg every 8 weeks. The study utilized drug-tolerant ELISA assay for UST trough levels and drug antibody titers.
Key findings:
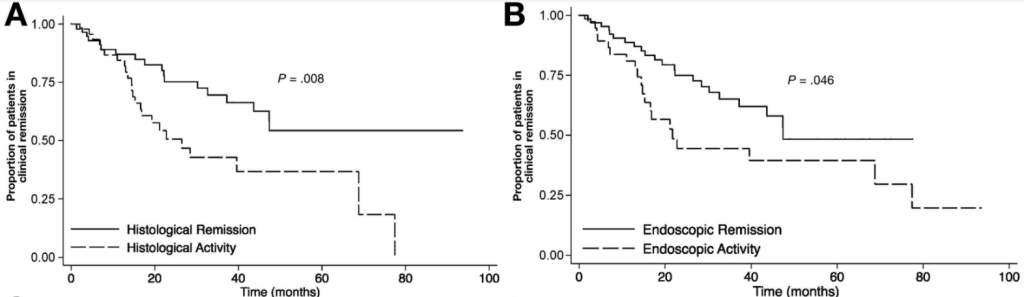
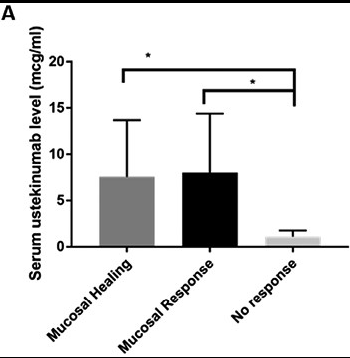
- In this observational cohort of patients with CD on maintenance UST, 63.8% of patients (n = 30 of 47) had achieved mucosal healing at time of level assay, and 85.1% (n = 40 of 47) had achieved at least mucosal response.
- Patients with mucosal healing (n = 30) had significantly higher mean serum UST levels (5.7 µg/mL, SD 6.4) compared with those with no response (1.1 µg/mL, SD 0.52; n = 7, P < .0001).
- Patients with mucosal healing (n = 30) had significantly higher mean serum UST levels (5.7 µg/mL, SD 6.4) compared with those with no response (1.1 µg/mL, SD 0.52; n = 7, P < .0001)
- Similarly, for patients with mucosal response (n = 40), we observed a higher mean serum UST trough level (5.1 µg/mL, SD 6.1) compared with those with no response (1.1 µg/mL, SD 0.52; n = 7, P < .0001).
- There were no antidrug antibodies detected in the cohort.
Discussion:
Unlike anti-TNF therapeutic drug monitoring, “there are few data supporting a correlation between serum ustekinumab levels and MH. The STARDUST randomized control trial34 is studying standard of care compared with treat-to-target ustekinumab therapy and has reported preliminary data at 1-year showing superiority of treat-to-target approaching achieving endoscopic response.”
My take: In this study, patients with UST levels above 2.3 μg/mL had a 10-fold level higher likelihood of mucosal healing and mucosal response. UST therapeutic drug monitoring can help “determine true nonresponse rather than insufficient dosing in patients who have not responded to UST.”
Other studies have suggested higher target levels. Mayo clinic lab site: “Concentrations > or =4.5 mcg/mL are associated with mucosal healing.” Ref: Papamichael K, Cheifetz AS, Melmed GY, et al. Appropriate therapeutic drug monitoring of biologic agents for patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2019;17(9):1655-1668.e3
Related blog posts:
- How Much Ustekinumab (Stelara) Is Needed to Get a Good Response This study show the need for higher levels of UST to achieve optimal outcomes. Levels of at least 8.4 (using Prometheus HMSA) appear to be a good target.
- Dose Escalation of Ustekinumab & Support Tool “Should I Have IBD Surgery?”
- Therapeutic Drug Monitoring: Ustekinumab (Stelara) (In 2017, this study suggested a level of at least 4.5)
- Expert Consensus: New Recommendations for Therapeutic Drug Monitoring
- Can You Give Ustekinumab Subcutaneously After IV Reaction?
- CCFA 2023 (Atlanta) Part 4
- CCFA 2023 (Atlanta) -Part 1