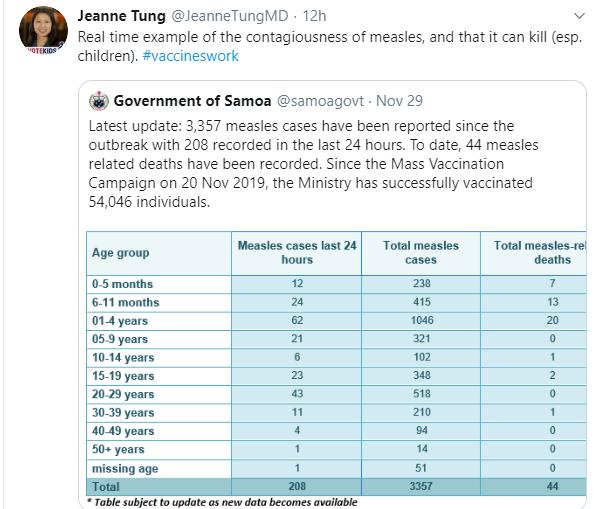
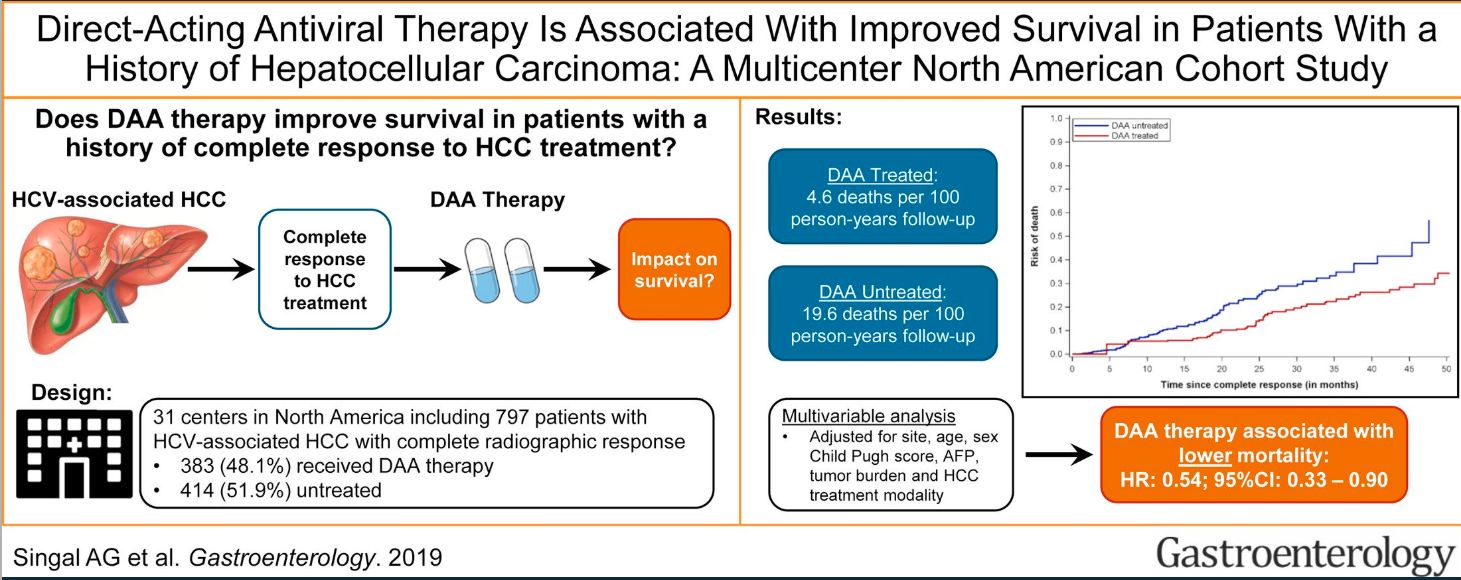
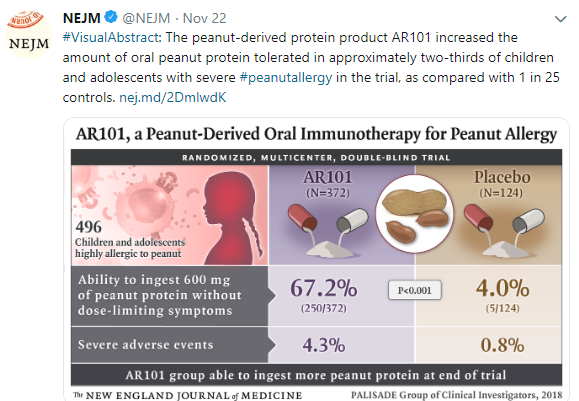
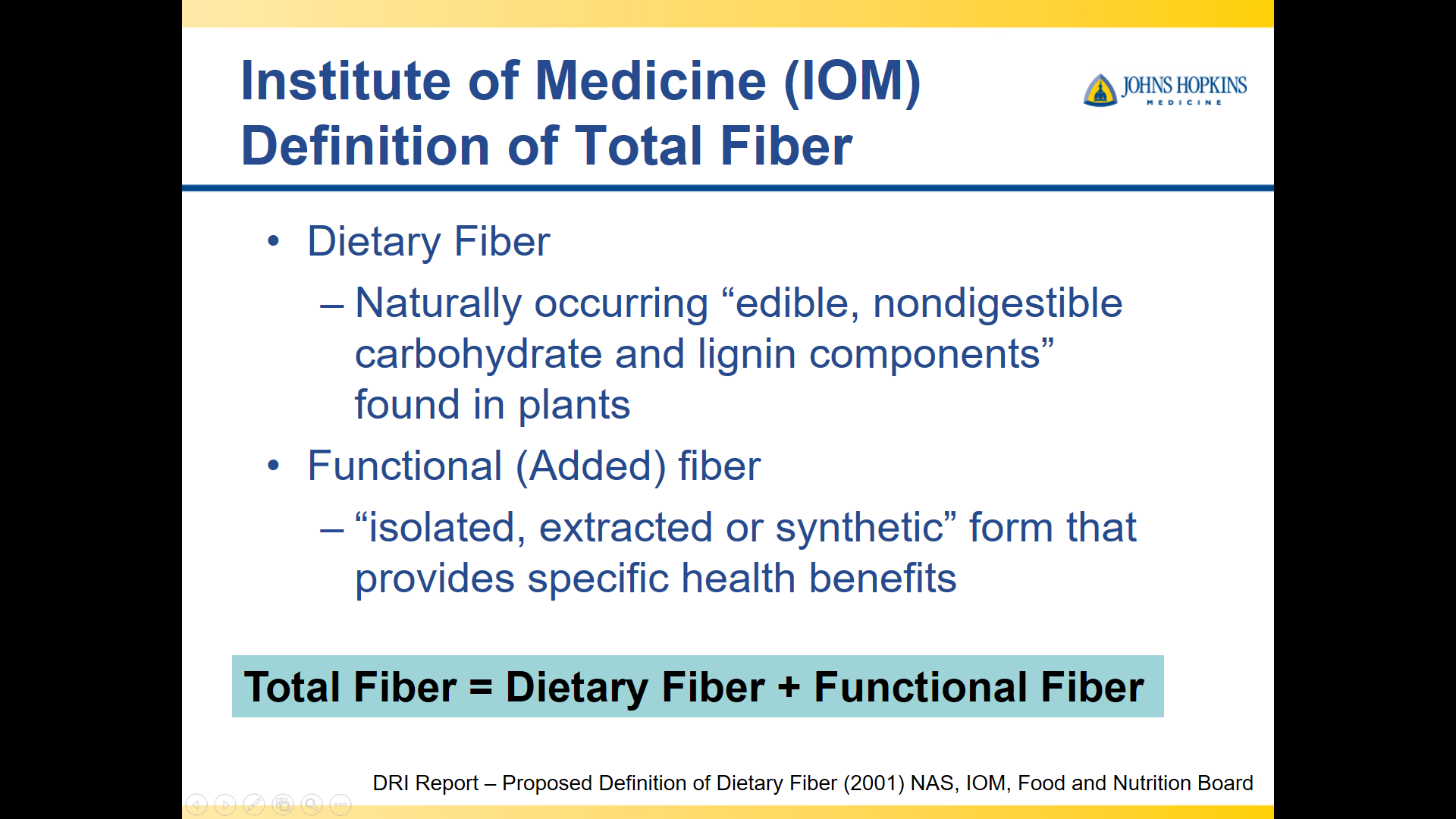
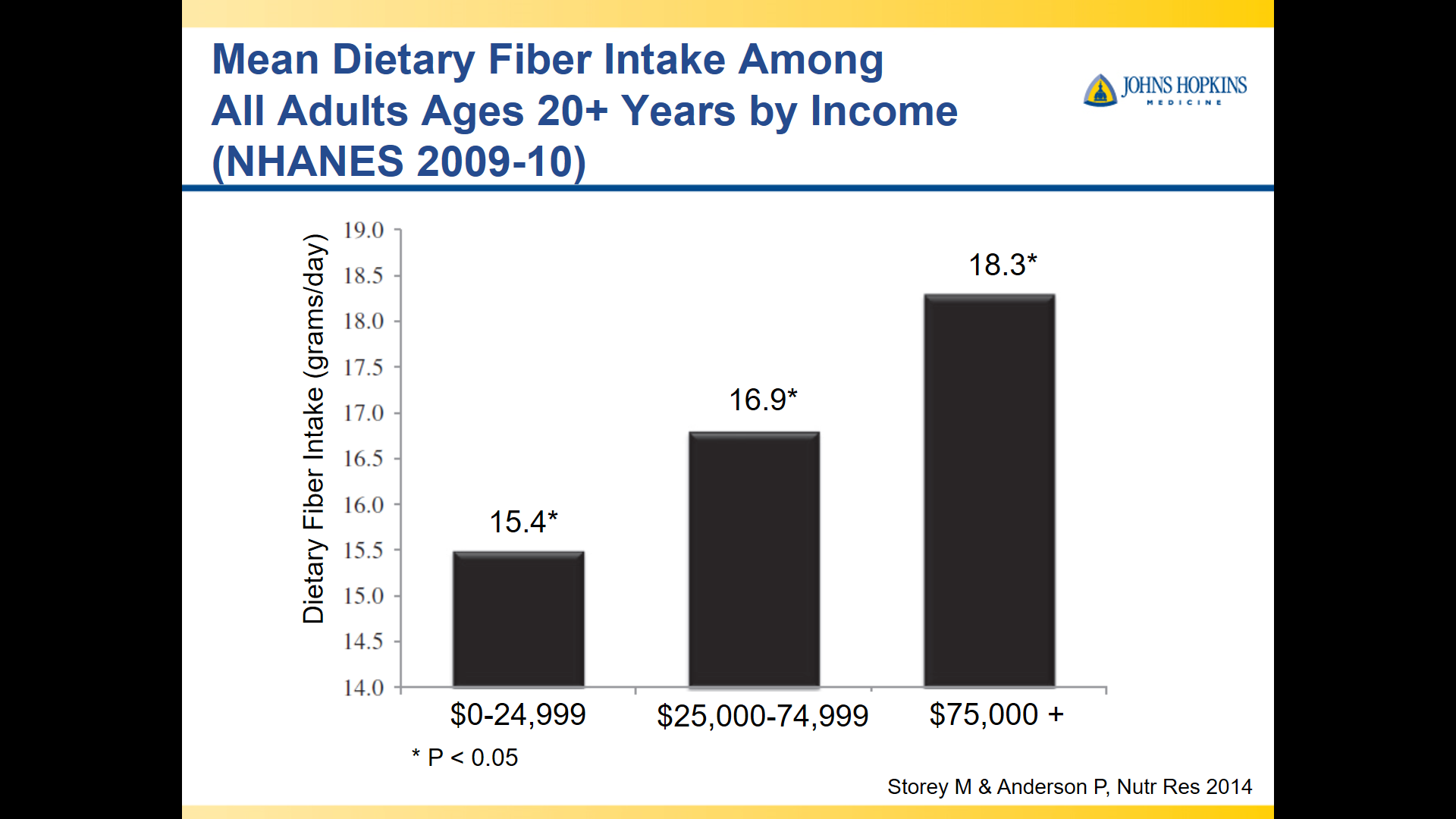
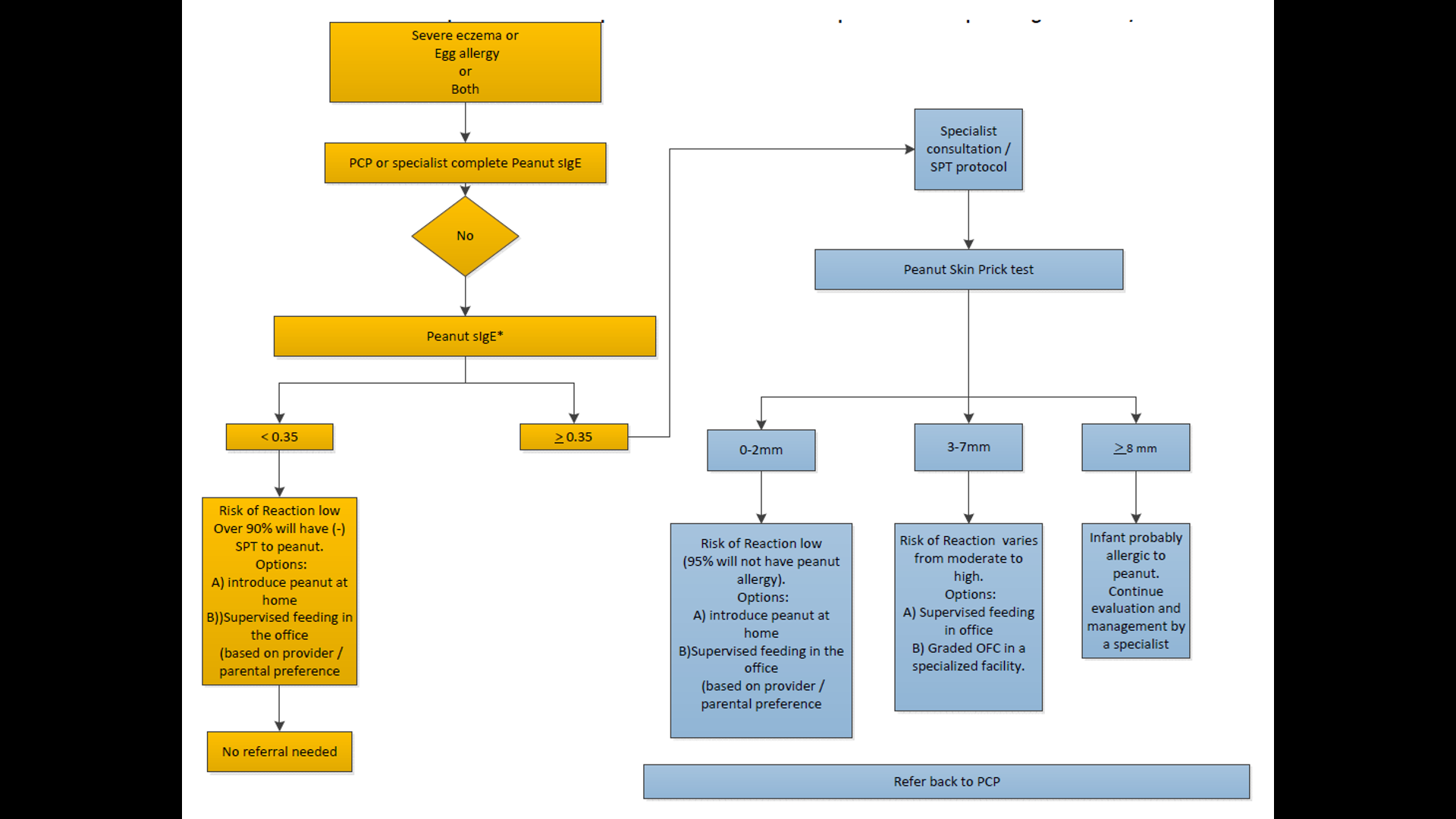
10/20/25 NY Times: Peanut Allergies Have Plummeted in Children, Study Shows “The new study, published Monday in the journal Pediatrics, found that food allergy rates in children under 3 fell after those guidelines were put into place — dropping to 0.93 percent between 2017 and 2020, from 1.46 percent between 2012 and 2015. That’s a 36 percent reduction in all food allergies, driven largely by a 43 percent drop in peanut allergies.”
Referenced article (Open Access!): S Gabryszeweki et al. Pediatrics e2024070516. Guidelines for Early Food Introduction and Patterns of Food Allergy
Related blog posts:
- Allergy Prevention Strategy: Early Exposure Beneficial (Long-term Data for Peanuts) (2024)
- The Peanut Story -From NEJM Blog
- “Separating Fact from Fiction in the Diagnosis and Management of Food Allergy”
- Peanut Allergy Prevention Guidelines
- LEAP-ON Study: Early Peanuts Prevent Allergies
- The Peanut Story -Skin Patch Chapter
- Links: Prevent Peanut Allergy (National Peanut Board)
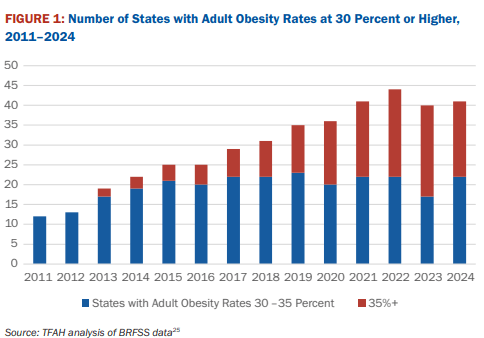
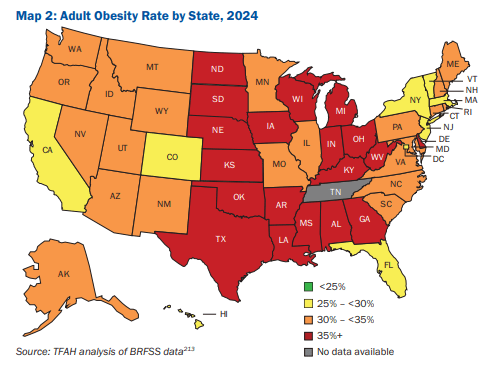
10/16/25 ABC News: Obesity remains high in the US., but more states showing progress, a new report finds “For the first time in more than a decade, the number of states with rates of obesity of 35% or more dropped, an encouraging sign that America’s epidemic of excess weight might be improving. But cuts to federal staff and programs that address chronic disease could endanger that progress, according to a new report released Thursday. Nineteen states had obesity rates of 35% or higher in 2024, down from 23 states the year before, according to an analysis of the latest data collected by the U.S. Centers for Disease Control and Prevention”
M Warren et al. Trust for America’s Health. Open Access! The State of Obesity 2025 Report (140 pages)
Related blog post: Worldwide Trends in Underweight and Obesity (2024)
10/13/25 NBC News: What to eat to ease chronic constipation, according to new guidelines This article revies the new dietary guidelines from the British Dietetic Association.

Related blog posts: