L Lara-Castor et al. BMJ 2024;386:e079234. Open Access! Intake of sugar sweetened beverages among children and adolescents in 185 countries between 1990 and 2018: population based study
Key findings:
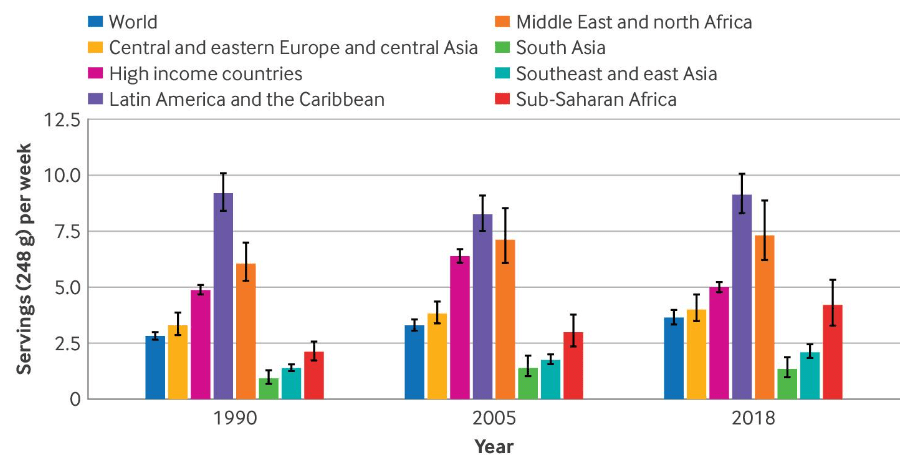
- Intakes of SSBs among children and adolescents aged 3-19 years in 185 countries increased by 23% (0.68 servings/week (0.54 to 0.85)) from 1990 to 2018, parallel to the rise in prevalence of obesity among this population globally
- High income countries experienced an overall decrease in intakes of SSBs from 2005 to 2018. This might be explained by the increasing scientific and public health attention on the harms of SSBs as well as obesity in these nations during this period, which may have led to increased media and public awareness about the harms to health associated with SSBs


My take: Despite the knowledge that sugary beverages are detrimental, consumption continues to increase.
Related blog posts: