S Singh et al. Clin Gastroenterol Hepatol 2023; 21: 2359-2369. Open Access! Comparative Safety and Effectiveness of Biologic Therapy for Crohn’s Disease: A CA-IBD Cohort Study
There is limited head-to-head data comparing the effectiveness of the biologics used for inflammatory bowel disease. In this study, the authors used a “series of propensity score (PS)-matched cohort studies comparing TNF-α antagonists vs vedolizumab vs ustekinumab in a large, diverse, multicenter, electronic health record (EHR)-based cohort.”
This graphical abstract summarizes the findings, though the first cohort (ustekinumab vs TNFalpha population is actually 1545 not 1030):
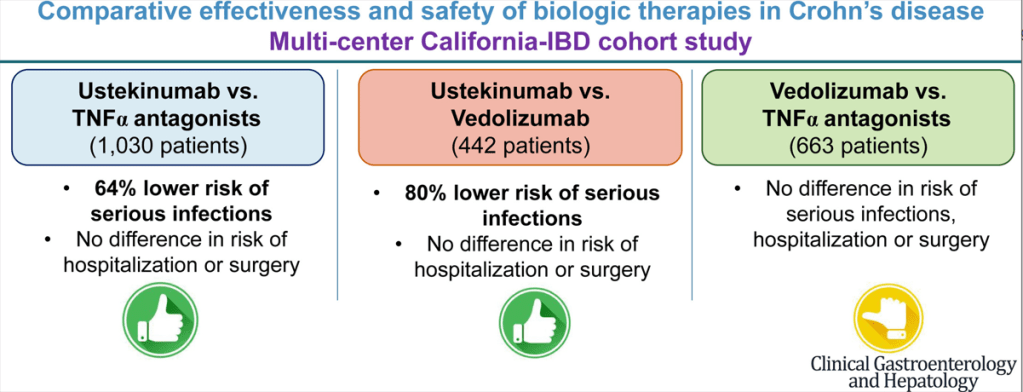
Key findings:
- Ustekinumab-treated patients with CD (n = 515) experienced a lower risk of serious infections (hazard ratio [HR], 0.36), without any difference in the risk of hospitalization (HR, 0.99) or surgery (HR, 1.08) -compared to patients receiving TNF alpha antagonists (n=1030)
- Ustekinumab-treated patients with CD (n = 221) experienced a lower risk of serious infections (HR, 0.20), without significant differences in risk of hospitalization (HR, 0.76) or surgery (HR, 1.42) -compared to vedolizumab-treated patients (n=221)
- Compared with TNF-α antagonists (n = 442), vedolizumab-treated patients with CD (n = 221) had a similar risk of serious infections (HR, 1.53), hospitalization (HR, 1.32), and surgery (HR, 0.63).
The increase rate of infections with vedolizumab compared to ustekinumab could be an indication of lower efficacy with vedolizumab as the medication itself has a high safety profile.
In the discussion, the authors comment further on head-to-head studies and lack of these as well. “Biemans et al23 observed that ustekinumab-treated patients were more likely to achieve corticosteroid-free clinical remission (69 patients in each arm, vs vedolizumab; 46.4% vs 29.0%; P = .04) and biochemical remission (42.1% vs 13.2%; P = .01) at 12 months, although these rates were not significant at earlier time points.”
My take: This study provides further evidence that ustekinumab is a good option for Crohn’s disease with regard to both safety and efficacy.
Related blog posts:
- Which is a More Effective First-Line for Crohn’s Disease: Ustekinumab or anti-TNF agents?
- Treatments for “Bad” Inflammatory Bowel Disease (Part 1)
- Head-to-Head (Sort of): Infliximab vs Ustekinumab for Crohn’s Disease
- Comparative Efficacy: Vedolizumab vs Anti-TNF Agents
- Vedolizumab vs Adalimumab: Histology Outcomes from Varsity Trial
- Ustekinumab for Refractory Pediatric Ulcerative Colitis and Updated Adalimumab Dosing
- IBD Updates: Understanding Newest IBD Therapies for Kids- Bowel Sounds, Hispanic Patients with IBD, More on Intestinal Ultrasound
