O Atia et al. Infammatory Bowel Diseases, 2025, 31, 617–624. Durability of the First Biologic in Children and Adults With Ulcerative Colitis: A Nationwide Study from the epi-IIRN
This was a nationwide Israeli study with 15,111 patients with UC, of whom 2322 (15%) received biologics, with a median follow-up of 7.0 years. The dataset includes ~98% of the Israeli population; “the accuracy of medication data is high, as the Israeli health care system provides medications almost free of charge through the HMOs, and the electronic dispensing of drugs contributes to reliable and precise data.”
Key findings:
- After 5 years of treatment, 43% of the patients with UC sustained their first biologic
- The durability rate was similar between pediatric-onset and adults after 1 and 5 years from initiation of treatment (72% and 43% vs 71% and 43%, respectively)
- Durability of adalimumab vs infliximab after 1 or 5 years was similar, whether prescribed as monotherapy (65%/46% vs 63%/33%, respectively) or combotherapy (78%/56% vs 91%/58%, respectively)
- Durability of infliximab at 1 yr and 5 yrs was higher as combotherapy (85%/50%) vs monotherapy (69%/42%; , P = .007), while it was similar for adalimumab (80%/52% vs 74%/52%)
- The durability rate was similar for vedolizumab monotherapy at 1 yr and 5 yrs (77%/56%) compared with adalimumab monotherapy (69%/52%), and infliximab monotherapy (73%/55% vs 62%/44%). However, combotherapy of antitumor necrosis factors (TNFs) had longer durability than vedolizumab (85%/50% vs 75%/43%), respectively;
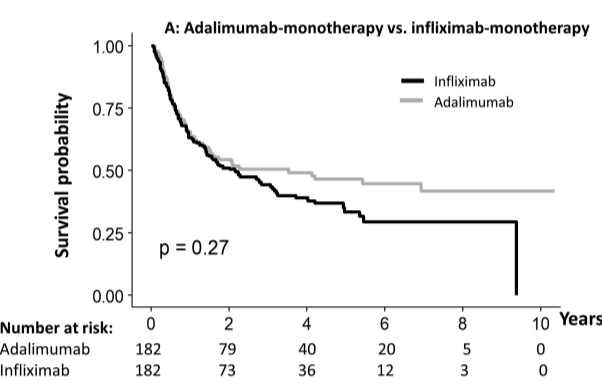
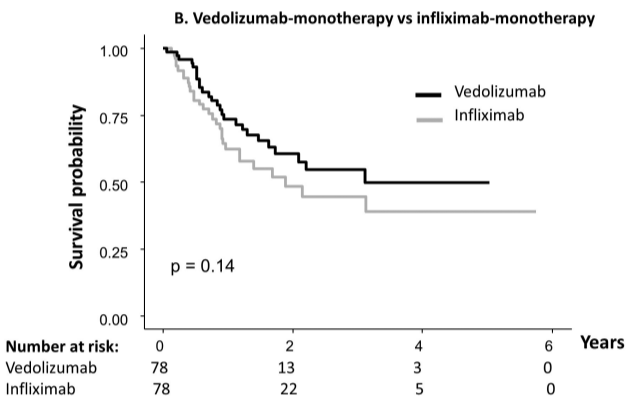
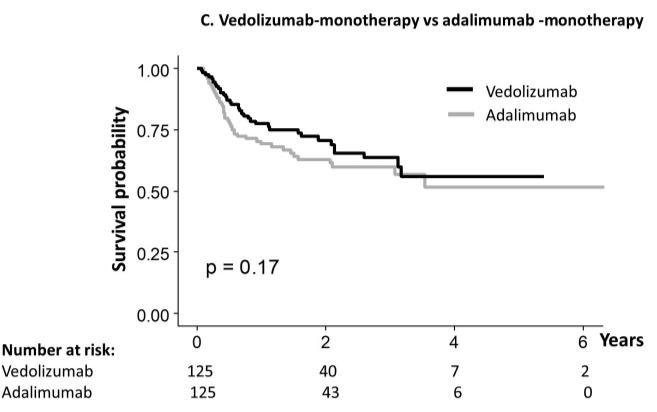
My take: When looking at the durability plots, the three main biologics in this study, infliximab, adalimumab and vedolizumab, performed similarly. Whether therapeutic drug monitoring would influence theses results is not clear. It is interesting that a recent study in the pediatric population found that combination therapy was important for adalimumab and not infliximab (see: Why Do Children Taking Adalimumab Benefit from Methotrexate Dual Therapy?)
Related blog posts:
- Comparative Evidence and Positioning Advance Therapies for Inflammatory Bowel Disease
- COMBO-IBD Study -Combination Immunomodulator Use and Thresholds
- Vedolizumab vs Adalimumab: Histology Outcomes from Varsity Trial
- Effects of Thiopurine Withdrawal in Randomized Trial of Vedolizumab-Treated Patients with Ulcerative Colitis
- Dr. Joel Rosh: Positioning Therapies for Pediatric Ulcerative Colitis
Also, from AGA Today (3/20/25): FDA Approves Guselkumab To Treat Patients With Crohn’s Disease
HCPlive (3/20, Campbell) reports the FDA on Thursday announced the approval of “guselkumab (Tremfya) for the treatment of adults with moderately to severely active Crohn disease.” The announcement from Johnson and Johnson claims the “approval is based on data from multiple phase 3 trials, including the GALAXI trials, which found guselkumab outperformed ustekinumab (Stelara) for multiple endoscopic endpoints. The agent now boasts indications for moderately to severely active Crohn disease and moderately to severely active ulcerative colitis (UC).” This is the fourth indication for guselkumab in the US
Related blog posts:
- Pivotal Study: Guselkumab Efficacy in Ulcerative Colitis (QUASAR study)
- IBD Updates: Insurance Barriers Hindering Care, Guselkumab vs Ustekinumab, IBD Pain Management Guidelines
- Guselkumab: Expanding the GALAXI of Treatments for Crohn’s Disease
- Dr. Maria Oliva-Hemker: Positioning Therapies for Pediatric Crohn’s Disease
Disclaimer: This blog, gutsandgrowth, assumes no responsibility for any use or operation of any method, product, instruction, concept or idea contained in the material herein or for any injury or damage to persons or property (whether products liability, negligence or otherwise) resulting from such use or operation. These blog posts are for educational purposes only. Specific dosing of medications (along with potential adverse effects) should be confirmed by prescribing physician. Because of rapid advances in the medical sciences, the gutsandgrowth blog cautions that independent verification should be made of diagnosis and drug dosages. The reader is solely responsible for the conduct of any suggested test or procedure. This content is not a substitute for medical advice, diagnosis or treatment provided by a qualified healthcare provider. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a condition.
