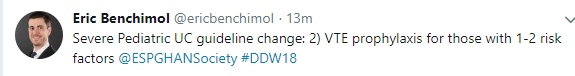With regard to yesterday’s post (Pediatric Guidelines for Ulcerative Colitis (Part 1)), the use of combination therapy with thiopurines is frequently avoided in the pediatric population in the U.S. due to safety concerns (eg. low risk of lymphoma). Anti-TNF monotherapy with pTDM appears to be a more common practice in the U.S. (Related blog post: Can Therapeutic Drug Monitoring with Monotherapy Achieve Similar Results to Combination IBD Therapy?). These pediatric guidelines with regard to combination therapy are similar to recent ACG guidelines for adults (D Rubin et al. The American Journal of Gastroenterology 120(6):p 1187-1224, June 2025. Open Access! ACG Clinical Guideline Update: Ulcerative Colitis in Adults).
————
A Assa et al. JPGN 2025; 81:816–85. Open Access! Management of paediatric ulcerative colitis, part 2: Acute severe colitis—An updated evidence-based consensus guideline from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition and the European Crohn’s and Colitis Organization
Comprehensive review (69 pages!) of all the topics related to acute severe colitis are covered. Topics include associated enteric infections (C diff, CMV), toxic megacolon, antibiotics, pain management, VTE, surgery, and pouchitis.
Some of the recommendations:
- All mesalamine preparations (oral and rectal) should be discontinued upon admission to exclude mesalamine intolerance, especially when mesalamine has been commenced during the preceding few weeks; (re-) introduction should be considered after significant improvement in the clinical condition [EL5, adults EL5] (*100% agreement).
- Regular diet should be continued in most ASC cases [not in toxic megacolon]. Enteral nutrition may be used if oral feeding is not tolerated or in malnourished children [EL4, adults EL1] (*100% agreement).
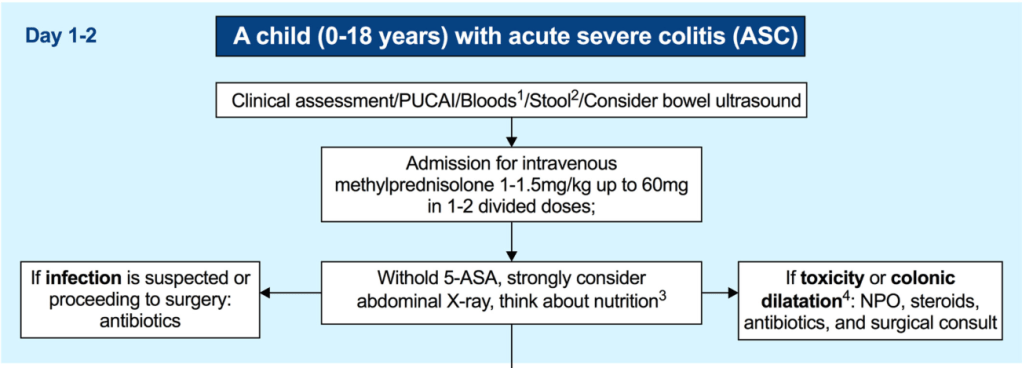
- Pharmacological thromboprophylaxis for reducing the risk of VTE should be considered in all inpatient children with ASC (Figure 1) [EL5, adults EL2] (*100% agreement).
- Intravenous methylprednisolone 1 mg/kg/day (up to 40 mg/day) once daily is the first-line treatment in ASC and should be promptly started [EL2, adults EL1]. A higher dose of 1.5 mg/kg/day (up to 60 mg/day) can be used at the clinician’s discretion (e.g., in patients on oral corticosteroids at admission and/or with a more severe spectrum of ASC) [EL4, adults EL4] (*100% agreement).
- Intravenous methylprednisolone should not be extended beyond 7–10 days of total course, since it carries no additional benefit and increases toxicity. In corticosteroid-refractory patients in whom second-line therapy is initiated, there is no need for corticosteroid tapering if corticosteroids are given as an isolated short course (up to 10 days) (*100% agreement).
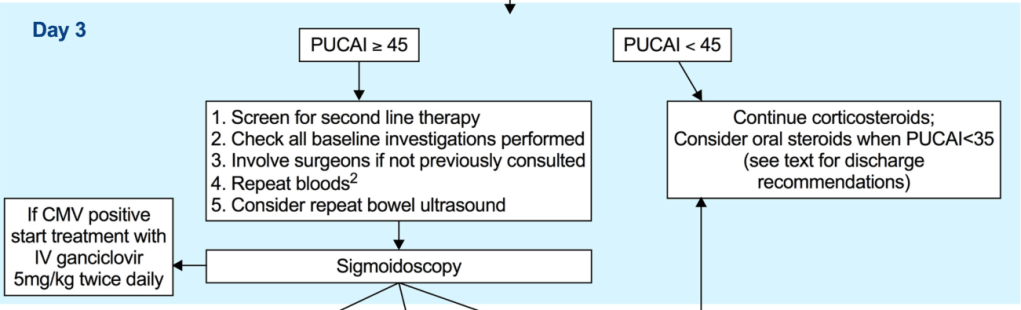
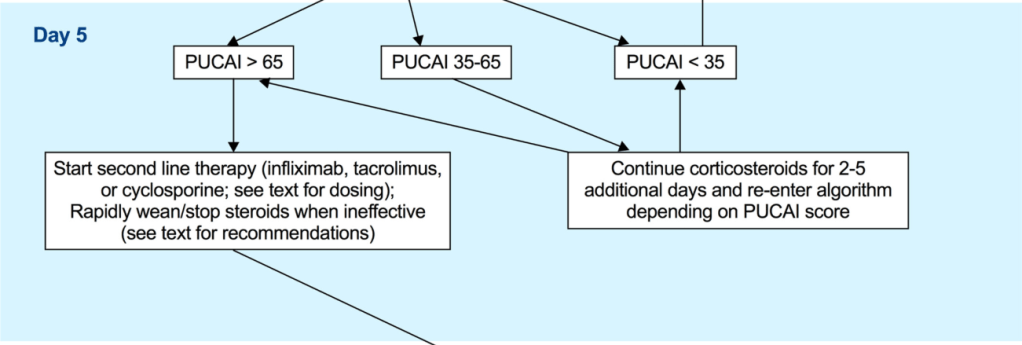
- A PUCAI > 45 on the 3rd day of IVCS treatment should dictate planning for second-line therapy between Days 3–5 [EL2, adults EL2] (*100% agreement).
- Second-line therapy should be initiated on the 5th day of IVCS treatment in children with a PUCAI ≥ 65 [EL2, adults EL2] (*100% agreement).
- Infliximab is recommended as the preferred second-line medical therapy for anti-TNF naive children failing IVCS [EL3, adults EL1] (*100% agreement).
- To reduce unnecessary immunosuppression, corticosteroids (when ineffective) should be rapidly weaned following introduction of second-line therapy or decision to proceed to colectomy (stopped if in use ≤10 days and reduced to prednisone ≤0.2 mg/kg or equivalent to 10 mg adult dose with gradual tapering thereafter if >10 days) [EL5, adults EL5] (*100% agreement).
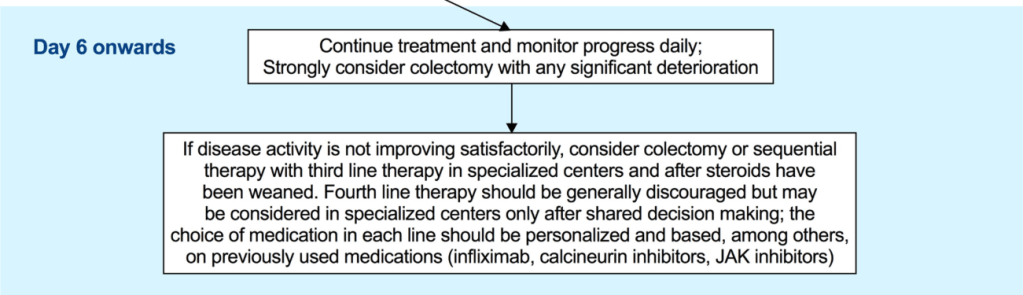
- Third-line sequential rescue therapies (CNIs after infliximab, infliximab after CNI or a JAK inhibitor after either) may be considered in stable patients, in specialised centres and in those whose corticosteroids were weaned off or nearly weaned off as stated above [EL5, adults EL2] (*100% agreement).
Related blog posts:
- ARCH Study: Higher Doses of Infliximab in Acute Severe Ulcerative Colitis
- “For Hospitalized Patients With ASUC, 5-ASA Adds No Value to Steroids”
- Early Assessment of Acute Ulcerative Colitis with ACE (Albumin, CRP, & Endoscopy)
- Management of Acute Severe Colitis
- AGA Guidelines: Moderate to Severe Ulcerative Colitis
- Management of Pediatric Ulcerative Colitis -ECCO Recommendations
- Accelerated Infliximab Dosing in Acute … – gutsandgrowth
- IBD Brief Updates: Anti-TNF Loss of Response, Upadacitinib for ASUC, Risk Factors for Developing IBD
Disclaimer: This blog, gutsandgrowth, assumes no responsibility for any use or operation of any method, product, instruction, concept or idea contained in the material herein or for any injury or damage to persons or property (whether products liability, negligence or otherwise) resulting from such use or operation. These blog posts are for educational purposes only. Specific dosing of medications (along with potential adverse effects) should be confirmed by prescribing physician. Because of rapid advances in the medical sciences, the gutsandgrowth blog cautions that independent verification should be made of diagnosis and drug dosages. The reader is solely responsible for the conduct of any suggested test or procedure. This content is not a substitute for medical advice, diagnosis or treatment provided by a qualified healthcare provider. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a condition.