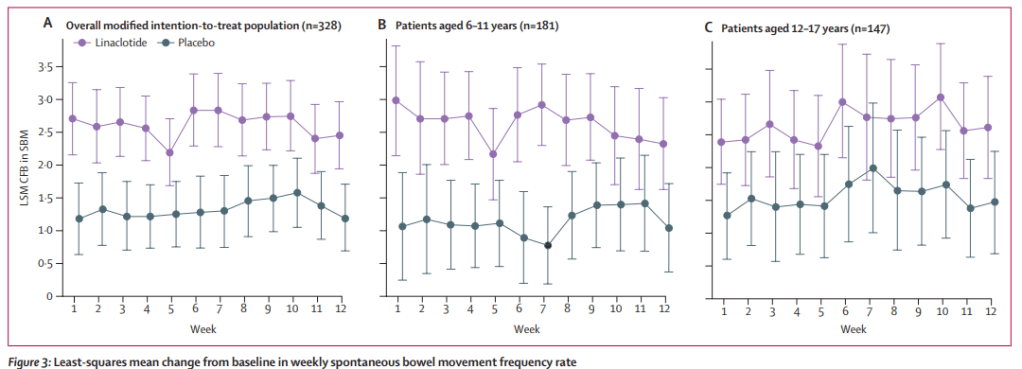
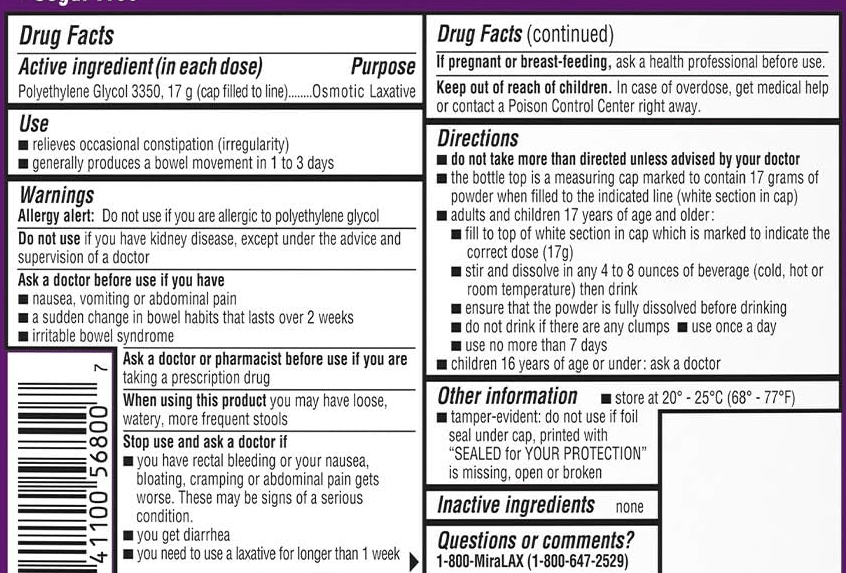
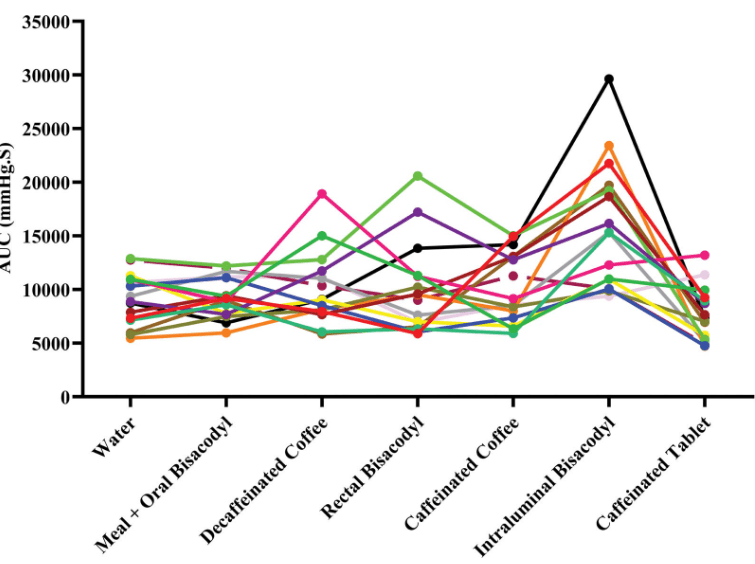
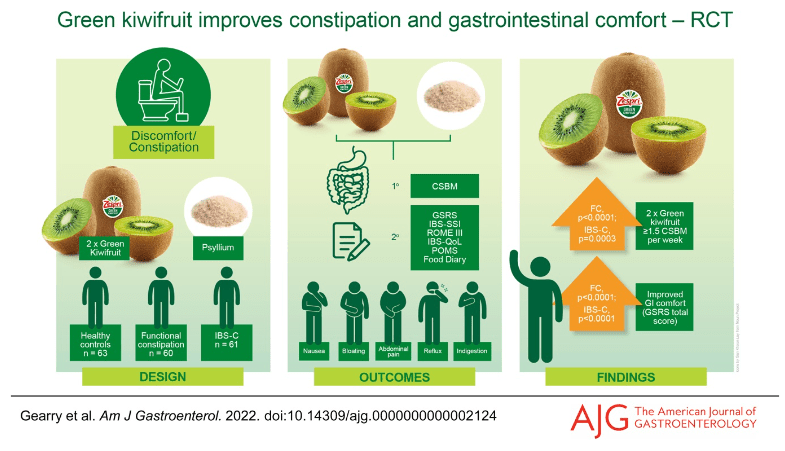
C-H Lo et al. Clin Gastroenterol Hepatol 2024; 22: 2309-2318. Open Access! Association of Ultra-processed Food and Unprocessed or Minimally Processed Food Consumption With Bowel Habits Among U.S. Adults
Methods: The authors used a cross-sectional study using data from the National Health and Nutrition Examination Survey (2005-2010) and they used two 24-hour dietary recalls and, based on the Nova classification, calculated intakes of ultra-processed foods (UPFs) and minimally-processed foods (MPFs). N=12,716 adults.
Key Findings:
- Median UPF and MPF intakes were 26.5% and 66.2% of total grams per day, respectively
- Greater UPF consumption (in % gram/d) was associated with higher odds of constipation
(adjusted OR [aORQ4 vs Q1], 2.20]
Discussion point: The authors did not find an association with diarrhea. “UPF consumption has been associated with increased risks of GI disorders that can cause chronic diarrhea including IBD and irritable bowel syndrome (IBS). This was thought to be related to alteration of the gut barrier integrity and activation of the immune response in the setting of microbial dysbiosis. The overall effect induces a pro-inflammatory micro-environment in the intestine and alterations in bowel function. However, the amount of UPFs needed to be
consumed by individuals such that the risk of diarrhea would be higher is unknown and likely varies between individuals.”
My take: Limiting UPFs and promoting fresh foods/minimally-processed foods is better for our health.
Related blog posts:
- Ultraprocessed Food and the Risk of Inflammatory Bowel Disease
- The Link Between Ultra-Processed Foods and Irritable Bowel
- AGA Guidance: Nutritional Therapies for Inflammatory Bowel Disease
- The Quality of Evidence for Dietary Treatments in Inflammatory Bowel Disease
- Risk Factors for Inflammatory Bowel Disease: Ultra-Processed Food (Part 1)