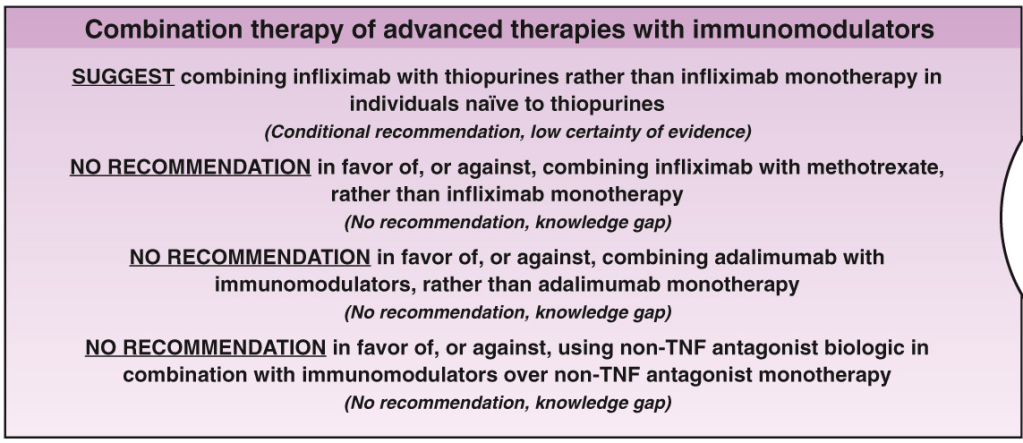
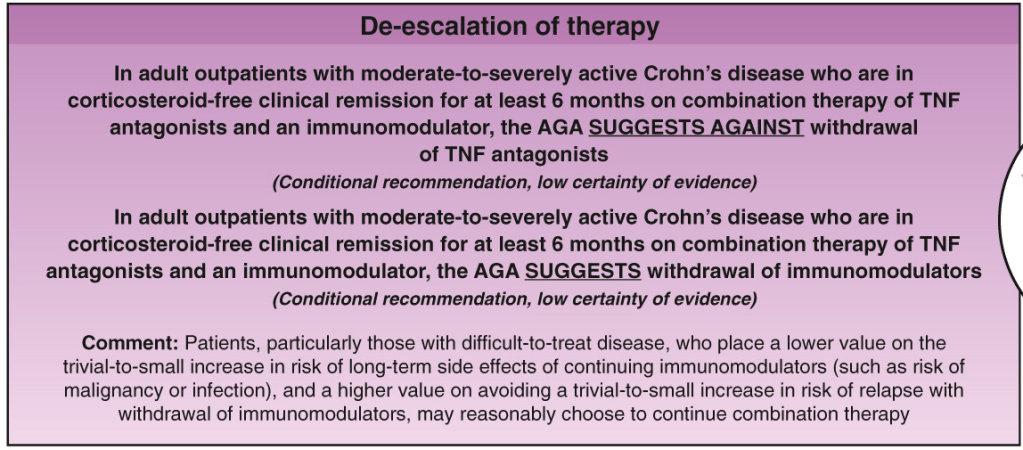
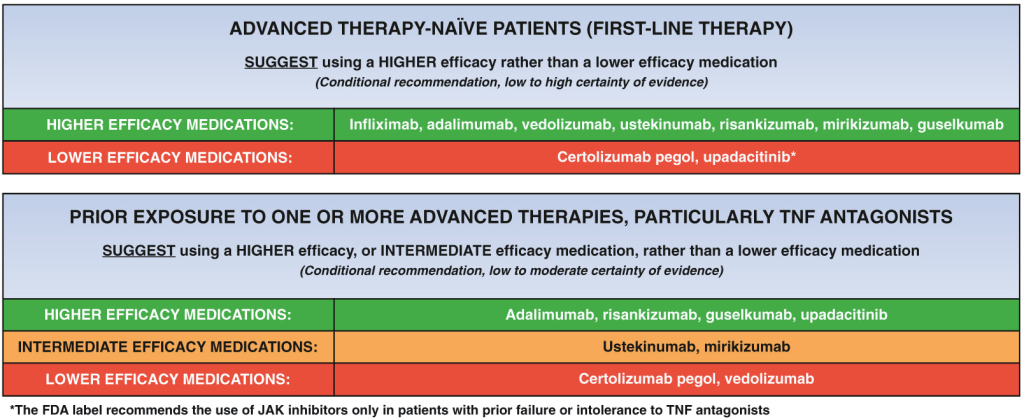
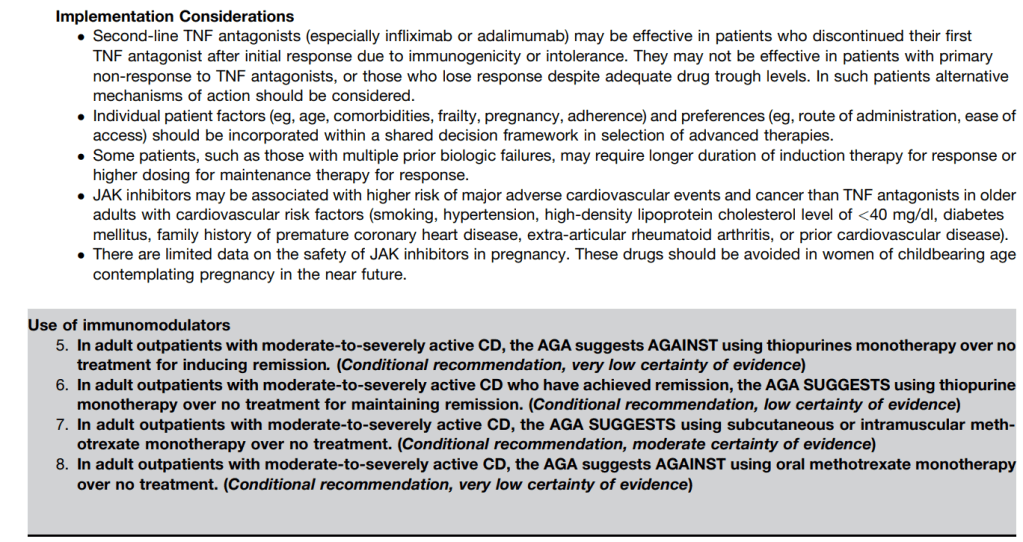
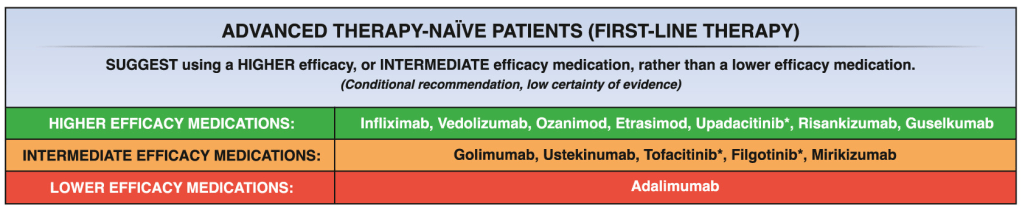
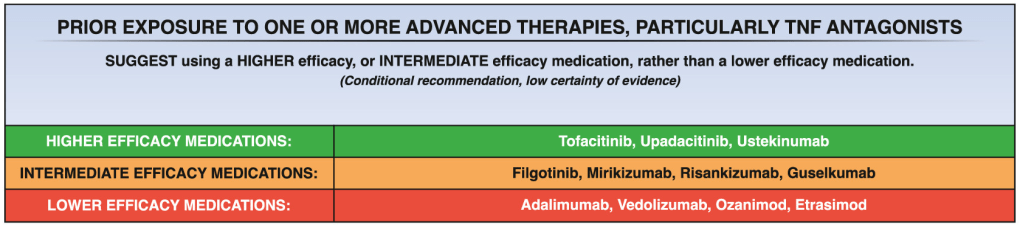
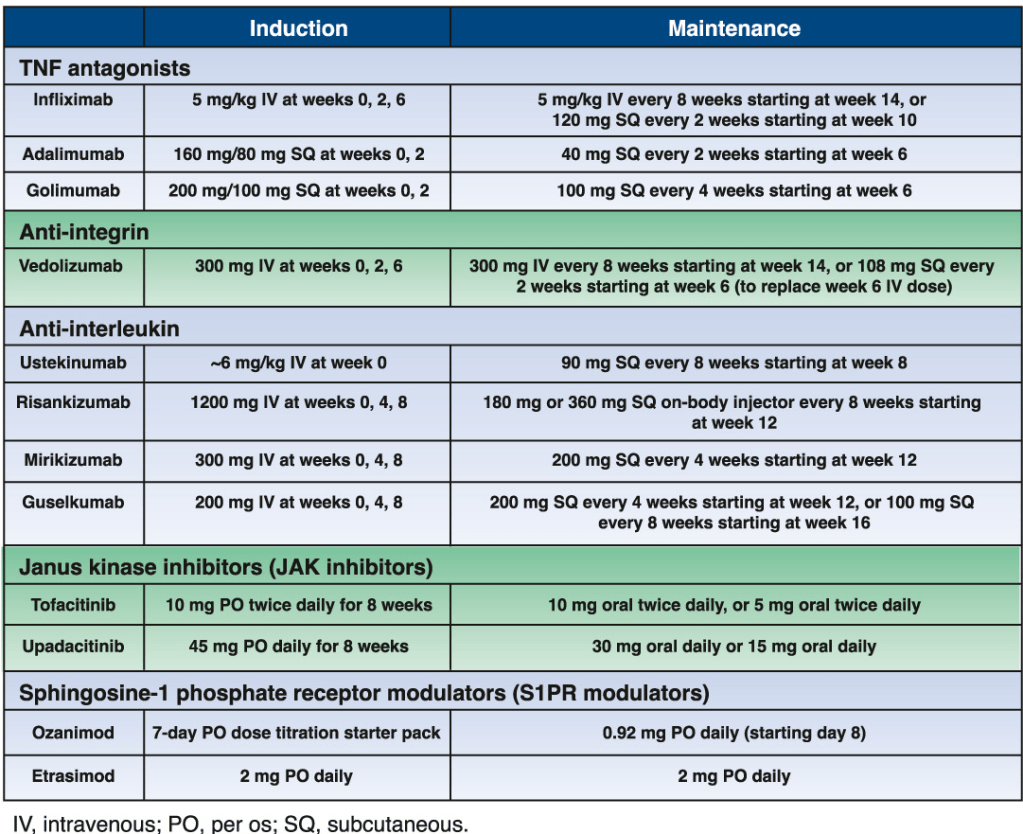
A useful recent article, ‘NASPGHAN Practice Guidelines for pediatric HCV’ (JPGN 2012; 54: 838-55) needs to be a handy reference. However, given the rapid changes in the HCV field, it is likely that this reference will need to be updated soon to incorporate new information (eg. IL28b) as well as emerging therapies.
Highlights:
Epidemiology: 0.2% of children & 0.4% of adolescents are HCV-infected; primary mode is mother to child (vertical) transmission which occurs in 5-7% if mother not coinfected with HIV
Testing: For infants of HCV-infected mothers, check HCV antibody after 18 months or HCV RNA at younger ages. Need two negative HCV RNAs to exclude infection (guidelines suggest checking 6 months apart). Most individuals should be screened with antibody testing and confirmed with RNA test.
Screening for HCC (U/S, AFP): suggested only “for those with significant liver disease (ie. cirrhosis)” due to rarity of HCC in pediatric HCC.
Treatment:
- Not if patient younger than 3 years
- Probably Pegylated-interferon with ribavirin –references for pediatric studies indicate response rates of about 50% for genotype 1 and about 80% for types 2 & 3.
- Who should be treated? Not always clear. Probably those with elevated aminotransferases or progressive disease based on liver biopsy. Possibly those with mild disease to eradicate virus.
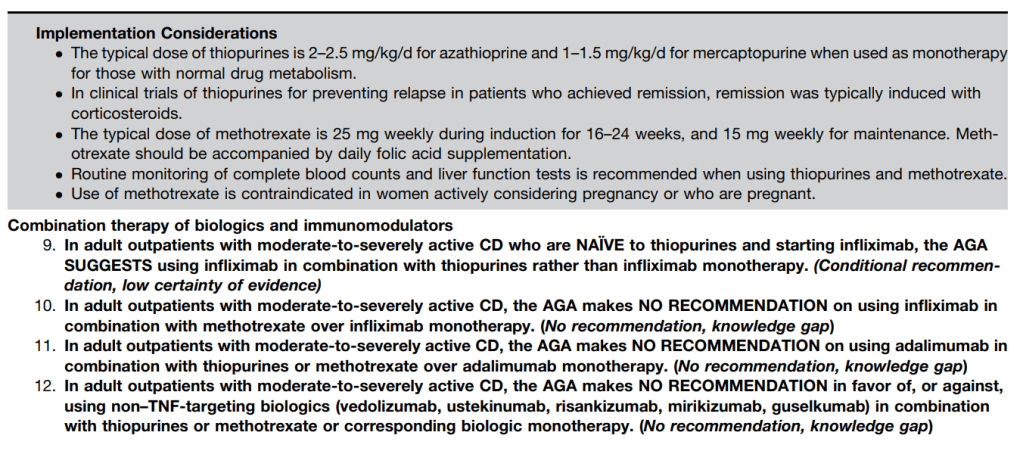
- Dosing: ribavirin 15/kg/day divided twice daily; weekly PEG-IFN-α-2a 180 microgram/1.73 m2 or weekly PEG-IFN-α-2b 60 microgram*m2
Treatment monitoring (Table 8):
- CBC/diff, Hepatic panel, glucose 0, 1, 2, 4, 8, 12 weeks, then every 4-8 weeks
- T4/TSH 0, 12, 24, 36, 48 weeks
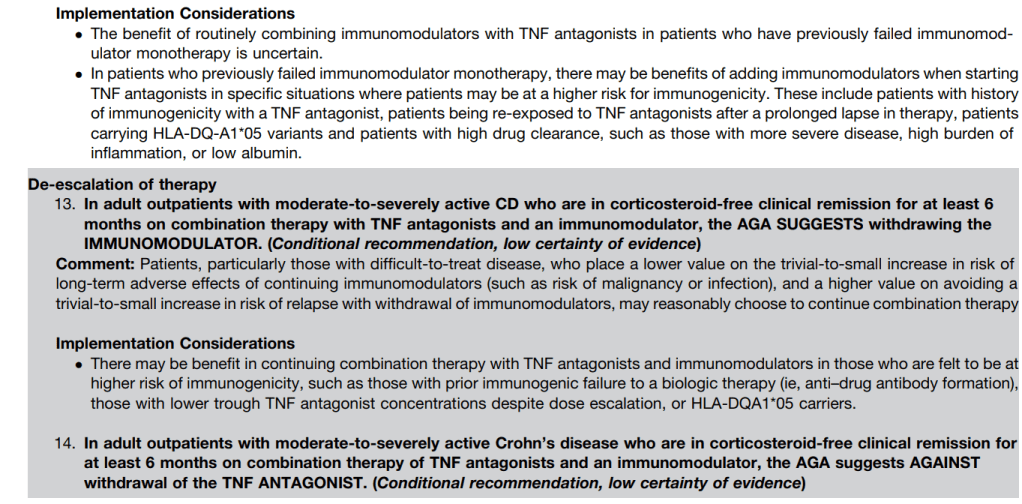
- Urine HCG 0, 24 weeks (if female >12 years)
- Prothrombin, Urinalysis at week 0
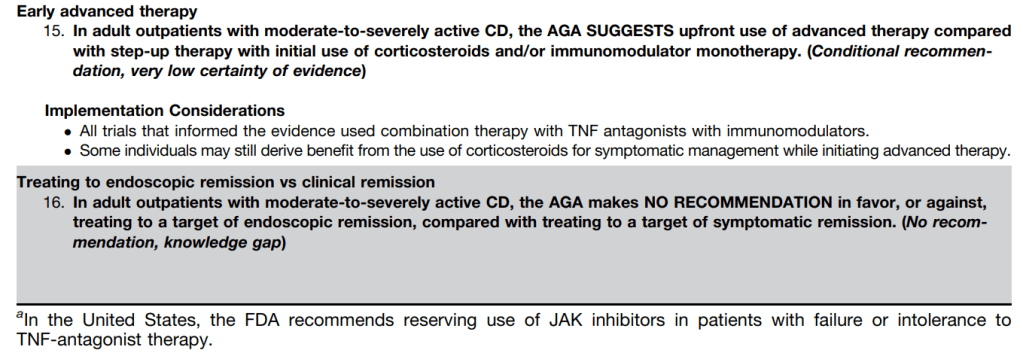
- HCV RNA 0, 24, 48, 72 weeks
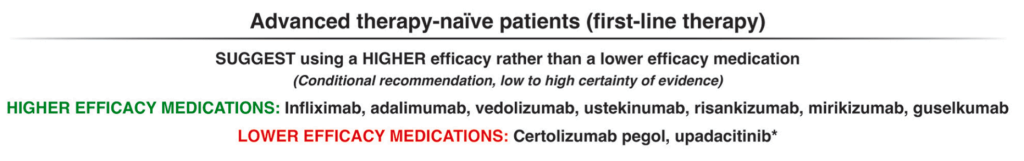
Anticipatory Guidance: “no legal requirement” to disclose HCV infection in U.S.; however, CDC suggests revealing this information to sexual partners (http://www.cdc.gov/hepatitis/hcv/)
- Avoid sharing toothbrush, shaving equipment with household contacts, unprotected sexual activity with multiple partners, tattooing/piercing
- Do not need to screen household or casual contacts
Special issues:
- Vaccines: HCV patients should receive all standard vaccines
- Obesity and alcohol both can worsen the outcome
- Fetal scalp probes and prolonged rupture of membranes but not route of delivery may increase risk of HCV transmission
- Breastfeeding is not contraindicated but should be avoided during mastitis/bleeding
Additional related blog links:
HCV now more deadly than HIV
The cost of progress in treating Hepatitis C
Increased ferritin predicts poor response in Hepatitis C
Curing Hepatitis C without interferon
Looking for trouble
Additional references:
- –Hepatology 2011; 54: 1433. AASLD guidelines. See teleprevir & boceprevir as well.-http://www.aasld.org/eweb/docs/hepatitisc
- -Hepatology 2011; 53: 1468. PEG/RBV have minimal effect on QOL/cognitive/emotional outcomes, n=114.
- -Gastroenterology 2011; 140: 389, 450-58. HEP-C STUDY. Comb RBV (15mg/kg div BID) & PEG-2a (180mcg/1.73m2 body surface q week) is better than PEG monotherapy. 53% SVR in combo group. Neutropenia in 40% –needed to reduce dose see below). “The Combination of Ribavirin and Peginterferon Is Superior to Peginterferon and Placebo for Children and Adolescents With Chronic Hepatitis C.”
- -Hepatology 2009; 49: 1335. Comprehensive review and guidelines
- -J Hepatology 2010; 52: 501-07. n=107. Pediatric study. Wirth et al. Efficacy of PEG alfa-2b (1.5/g/d) & RBV (15/kg/day): Genotypes 2/3 96% SVR, genotype 1 55%.
- -JPGN 2006; 43: 499. Study of PEG-IFN-α-2a in children. dose BSA m2/1.73 x 180microgm weekly x 48 weeks. 6/14 (43%) had sustained response. all genotype 1. Article states that IFN (3/week) + RBV has now been approved by FDA for those over 3 years
PEG-Interferon Dosing:
Dosing adjustment from hep C study in children –needed in ~40%
PEG -2a
original: 180mcg/1.73m2
1. level 1: 135 mcg, level 2: 90mcg, level 3: 45 mcg
If ANC 750-999 week 1-2: level 1 adjustment, weeks >3: no adjustment
If ANC 500-749: week 1-2: hold dose ’til >750, then level 1; weeks >3, level 1 adjustment
If ANC 250-499: week 1-2, hold until >750, then level 2 adjustment, weeks >3, then hold ’til 750, then level 1 adjustment
If ANC <250, stop drug
If PLTs 35-49K, hold til >50, then level 1
If PLTs 25-34, hold til >50, then level 2
If PLTs <25, stop drug
If Hgb <10, reduce RIBA dose by 1/2 & increase dose when hgb>10
If hgb <8.5, stop RIBA
If indirect bili >5, stop drug. If <2.5, restart dose at one-half and if remains less than 2.5, can resume full dose after 4 weeks.
IF ALT 5-10 ULN, recheck in 1 week. If stays high, level 1 adjustment.
IF ALT10 ULN for more than 1 week, then if drops to 5-10, level 1 but if remains >10 ULN, then stop drug.
Side effect frequency:
flu symptoms: 91%, h/a, 62%, GI symptoms 56%, injection pain 45%, muscle aches 36%, irritable 31%, fatigue 27%, rash 20%, itching 15%, anorexia 13%, trouble sleeping 11%, depression 4-12%