M Ferrante et al. The Lancet 2024; https://doi.org/10.1016/S0140-6736(24)01762-8. Efficacy and safety of mirikizumab in patients with moderately-to-severely active Crohn’s disease: a phase 3, multicentre, randomised, double-blind, placebo-controlled and active-controlled, treat-through study
Methods: VIVID-1 was a global phase 3, randomized, double-blind, double-dummy, placebo-controlled and active-controlled, treat-through study which enrolled 1150 patients with moderate-to-severe Crohn’s disease. There were three treatment groups: mirikizumab group, ustekinumab group, and placebo group. In each group, 48-49%were considered “biologic-failures” including 45-46% who were anti-TNF failures.
Key findings:


Discussion points:
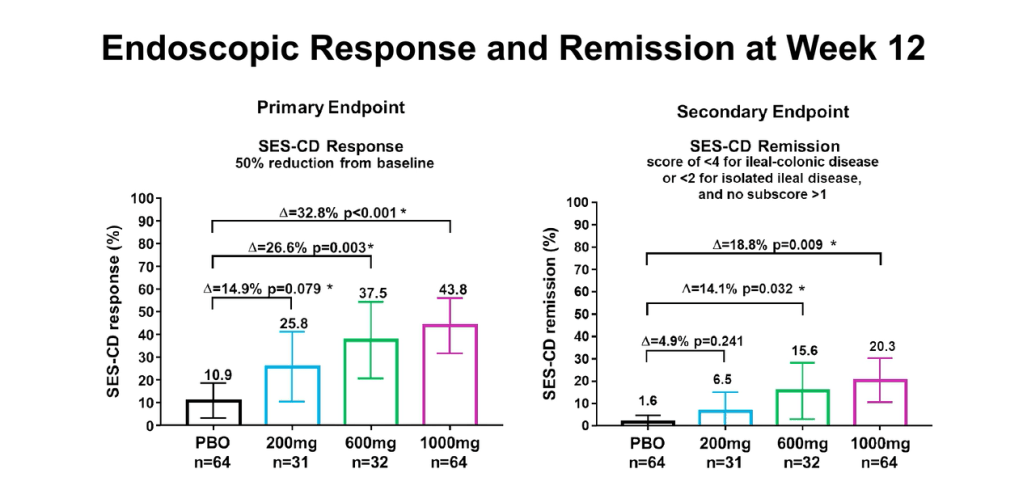
Early treatment effect: “Symptomatic improvement was evident as early as week 4 accompanied by a statistically significant reduction in high-sensitivity CRP and faecal calprotectin, and endoscopic response was seen at week 12.”
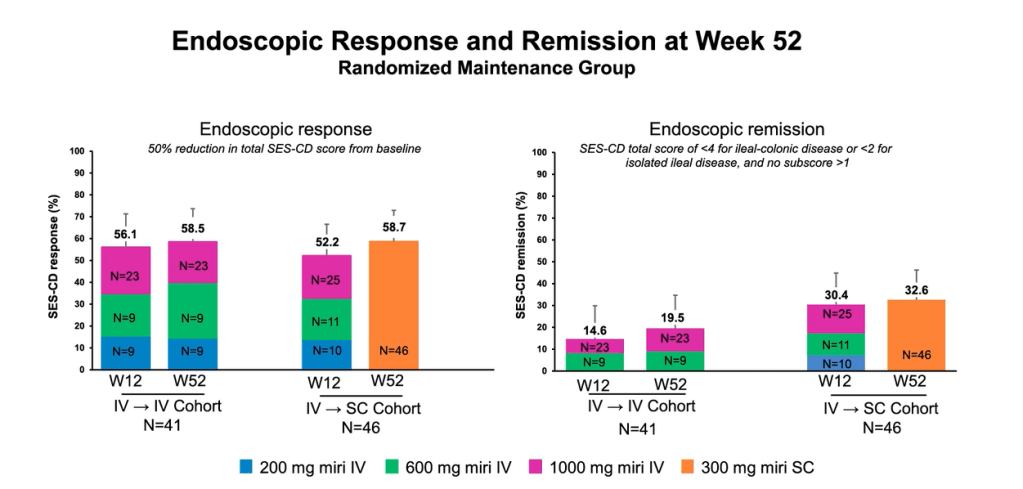
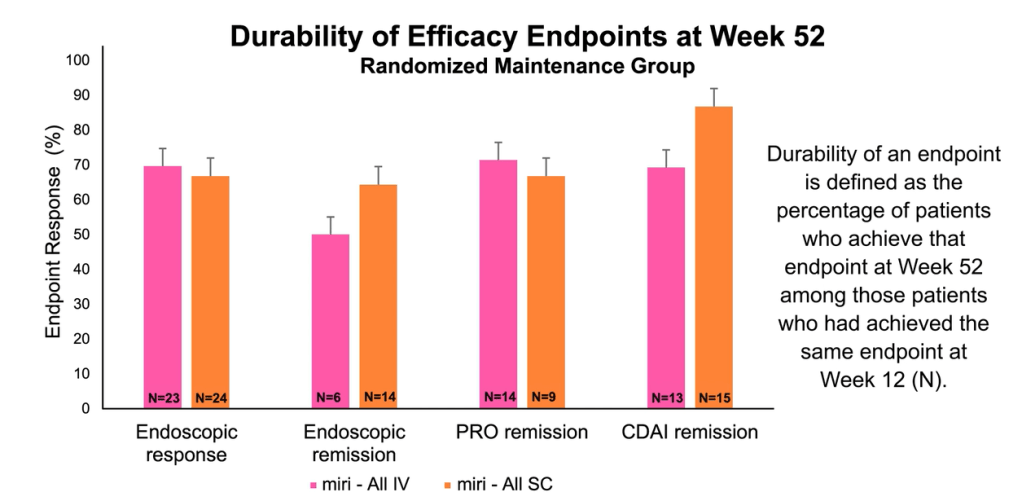
Compared to ustekinumab: “Mirikizumab reached non-inferiority versus ustekinumab for clinical remission by CDAI at week 52…mirikizumab showed statistically significantly greater improvements from baseline in fecal calprotectin and CRP compared to ustekinumab.
In addition, a greater percentage of patients reached the combination endpoint of endoscopic response and clinical remission by CDAI at week 52.”
Comparison across treatment trials: “. At week 52, 45∙4% of patients treated with mirikizumab met the endpoint of clinical remission by CDAI in the treat-through analysis with composite endpoint, 54∙1% met the endpoint in the treat-through analysis, and 64∙3% met the endpoint in the responder analysis. This example, with a range of nearly 20% percentage points depending on analysis type, shows the profound limitations in comparing
unadjusted outcomes across phase 3 trials.” The authors note other differences in trial design between VIVID-1 and SEQUENCE (risankizimab) and state “no conclusions on
relative efficacy can be drawn.”
My take: This study shows that mirikizumab is effective in adults with moderate-to-severe Crohn’s disease with and without prior biologic treatments. Pediatric studies are underway.