- R Bissonnette et al. NEJM 2025; 393: 1784-1795. Oral Icotrokinra for Plaque Psoriasis
- RS Stern. NEJM 2025; 303: 1854-1855. Oral Psoriasis Therapy — For Whom and at What Cost and Risk?
- S Wharton et al. NEJM 2025; 303: 1796-1806. Orforglipron, an Oral Small-Molecule GLP-1 Receptor Agonist for Obesity Treatment
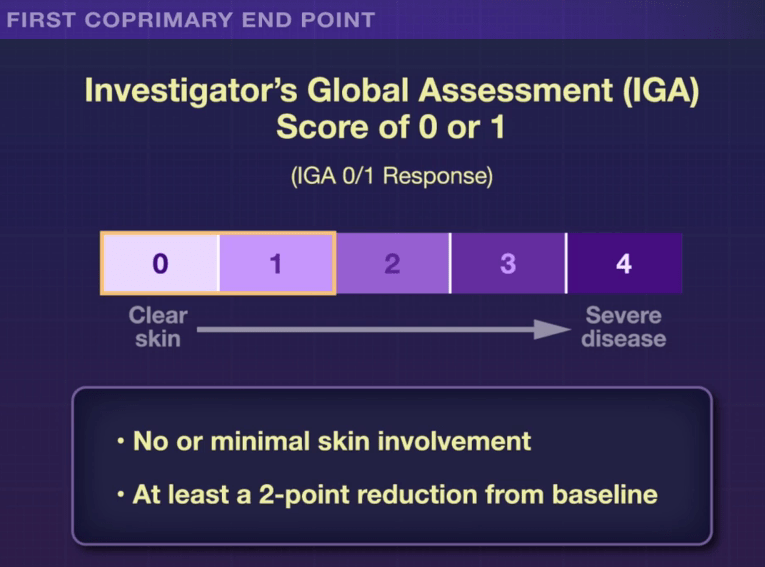
In the ICONIC-LEAD study (Bissonnette et al), 684 adolescents and adults participated in a DBPC trial with an oral peptide, icotrokinra, which binds the IL-23 receptor. This medication is of interest as there are ongoing trials with it for inflammatory bowel disease. Other injectable medications targeting IL-23 are already approved for IBD.
Key Findings:
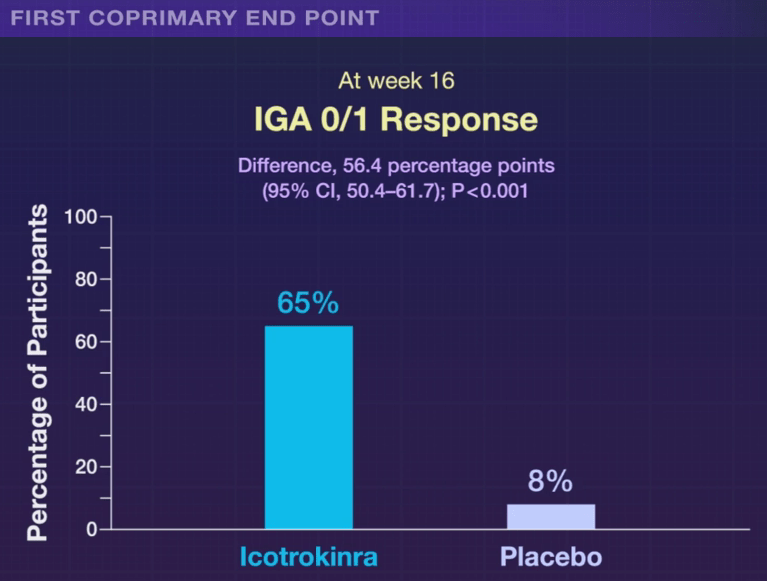
The associated editorial notes that this new therapy is likely to cost ~$70,000 per year. The cost of psoriasis care has increased more than 2000% since 1997. “Because of these high prices, rebates and discounts to pharmacy benefit managers that often guide formulary preferences are likely to govern clinician’s selection of immune-based oral and parenteral therapies for psoriasis.”
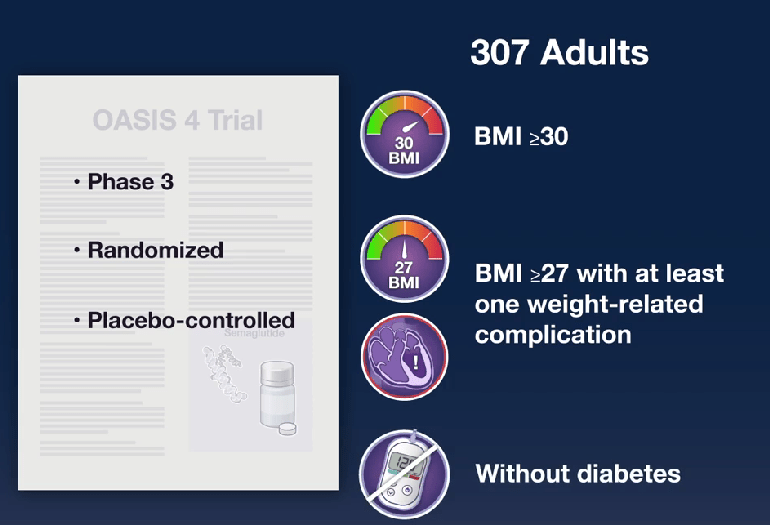
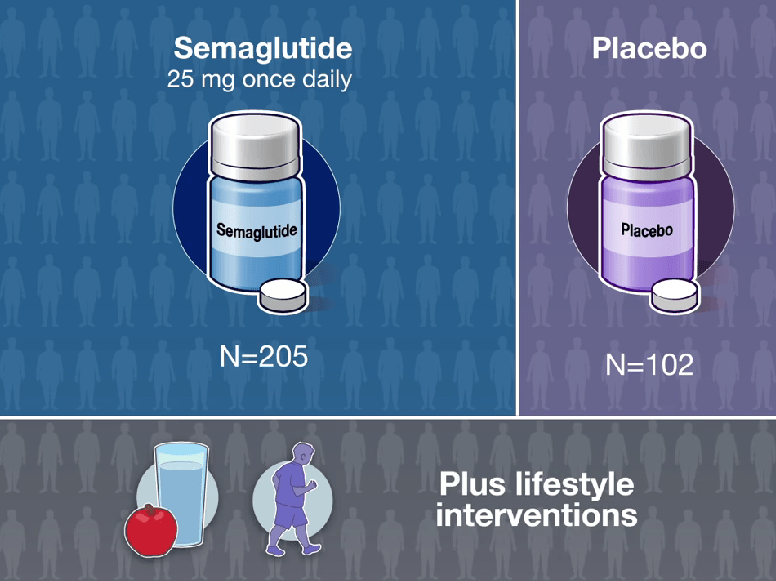
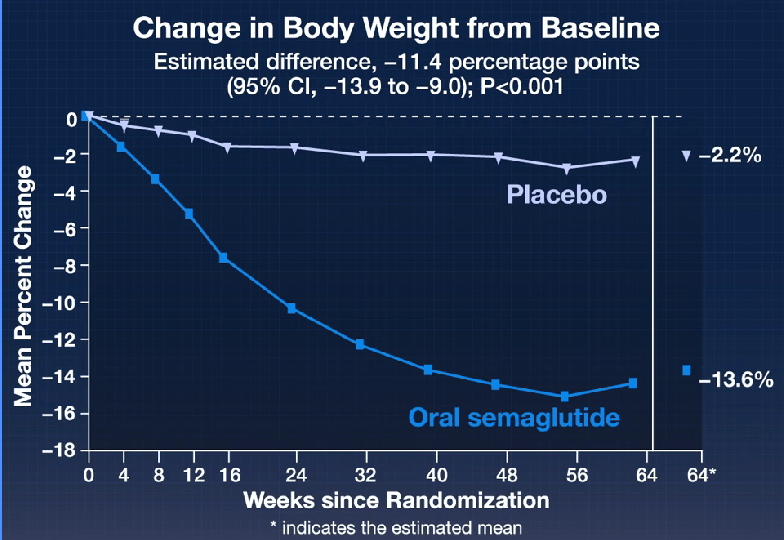
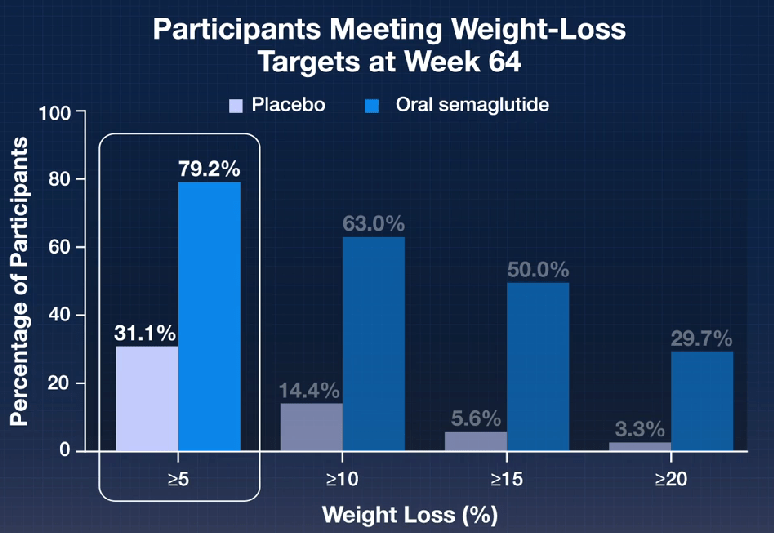
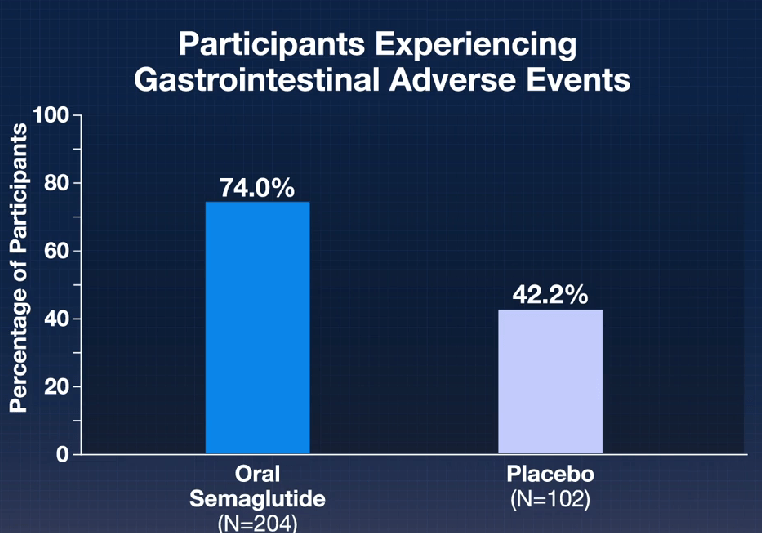
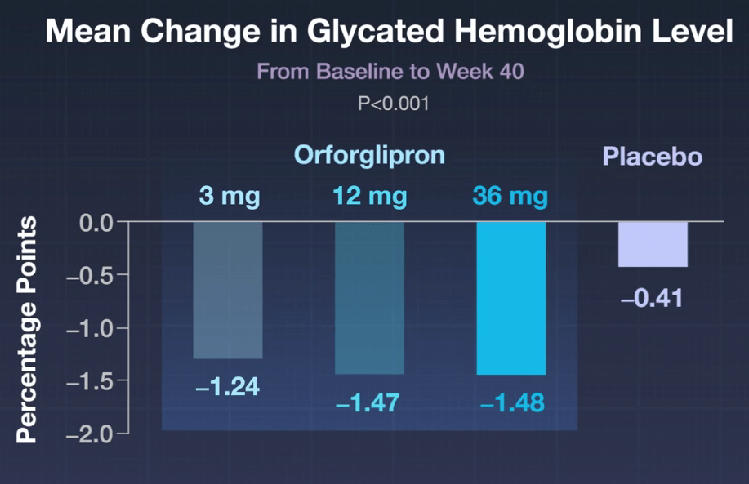
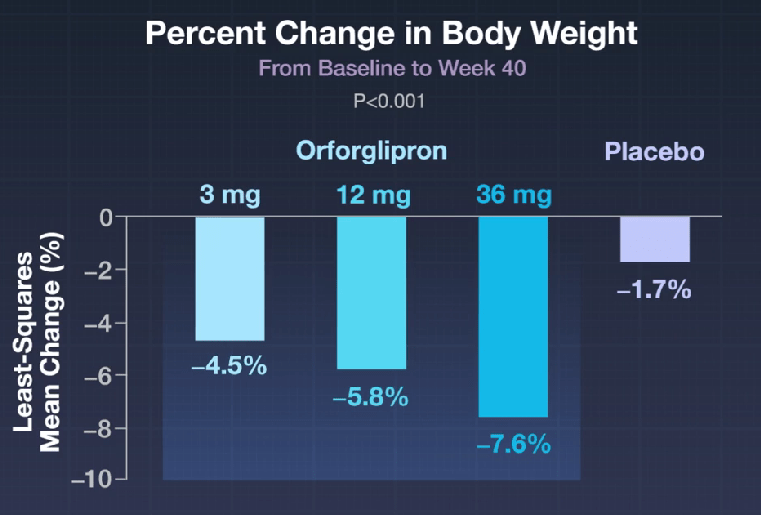
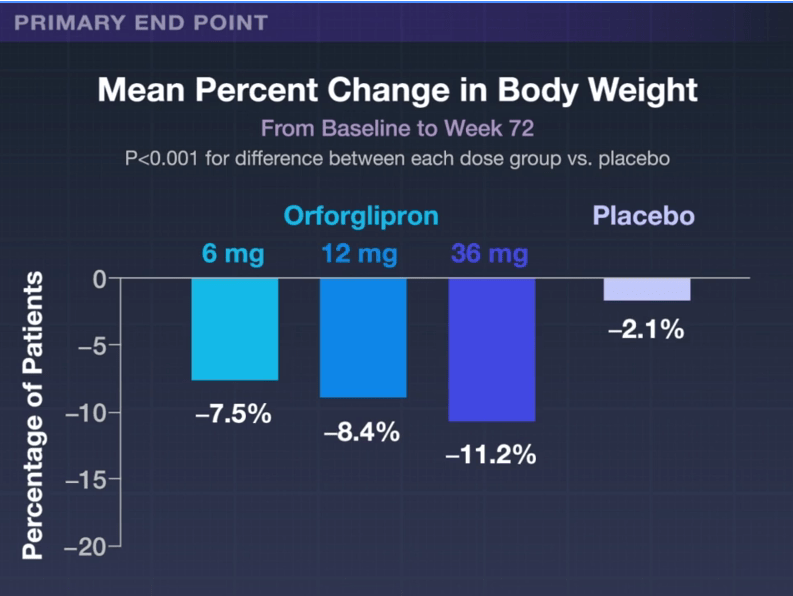
In the ATTAIN-1 Trial (Wharton et al), the authors share the results of an oral GLP-1 Receptor Agonist, Orforglipron, monotherapy for obesity.
Key findings:
- Georgia Tech Newsletter, 11/8/25. These ‘Exploding’ Capsules Could Deliver Insulin Without a Needle

My take: There are similar injectable alternatives to each of these medications for psoriasis, obesity and diabetes. The availability of oral medications could reduce one barrier to treatment. Cost barriers may preclude their use in many patients when they become available. In addition, long-term outcome data are still needed.
Related blog posts: