K Karrento et al. J Pediatr Gastroenterol Nutr. 2025;80:1028–1061. Open Access! North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition 2025 guidelines for management of cyclic vomiting syndrome in children

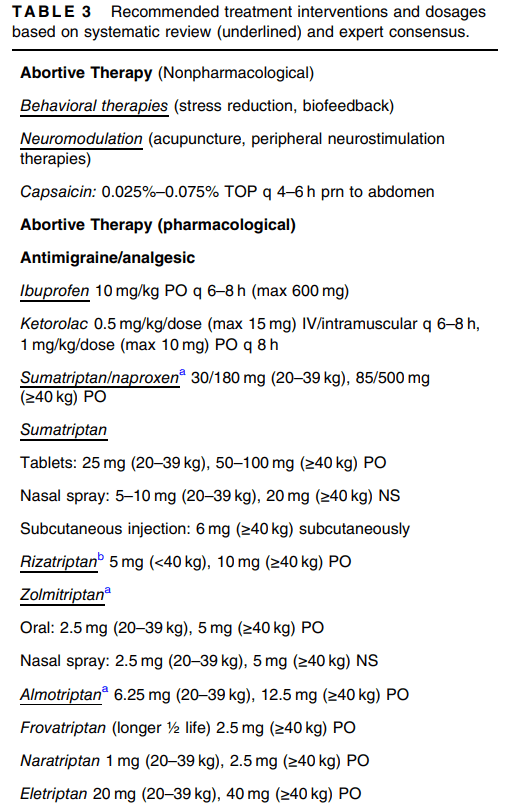
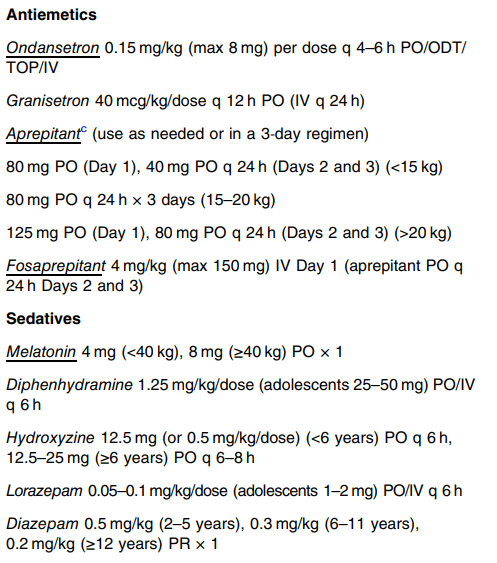

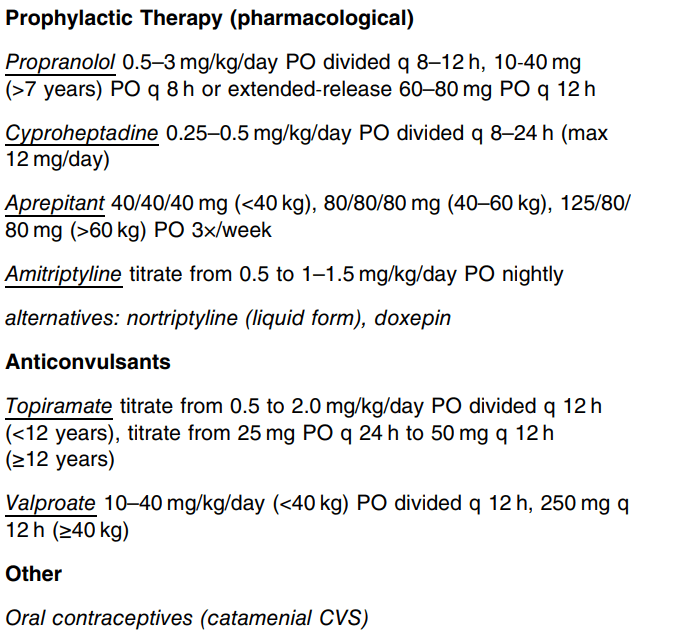
This is an excellent CVS guideline (36 pages). There is a lot of information and advice that is not easily summarized. Some important points:
- Epidemiology: “The prevalence of pediatric CVS is estimated between 1.9% and 2.3% with an incidence of 3.2 per 100,000 children/year.1–3, 16 CVS peaks among school-aged children and often evolves into migraine headaches in adolescent years…17 56% of children experience resolution of CVS during a median follow-up of 29 months (range 6 months to 7 years)…A long-term follow-up study demonstrated progression to migraine in 26% of those with pediatric CVS.1“
- Autonomic dysfunction: “An underlying autonomic dysregulation is also supported by clinical features during attacks (diaphoresis, listlessness, palpitations, and peripheral vasoconstriction), and a study shows that 40% of pediatric patients with CVS develop chronic dysautonomia during adolescence.18“
- Cannabinoid hyperemesis syndrome: “CHS is considered a probable subtype of CVS that presents after prolonged and excessive cannabis use…26 Topical capsaicin, benzodiazepines, and droperidol or haloperidol have all been proposed as possible treatments for acute CHS episodes…50 Adult guidelines recommend that CHS patients be offered the same therapies as CVS patients…Complete cannabis cessation is the only known effective long-term treatment for CHS.”
- Sato-variant: “This subtype manifests elevated levels of adrenocorticotropin hormone, cortisol, antidiuretic hormone, catecholamines, and prostaglandin E2, consequently presenting with hypertension and profound lethargy.25 While there is no published data for guidance, electrolyte monitoring is warranted, and episodic hypertension is generally managed by short-acting agents such as lisinopril or labetalol.”
- L-carnitine: “The panel did not find evidence of efficacy other than when used in combination with coenzyme Q10 and cautioned against use based on concerns for atherosclerosis in animals.”
- Propranolol: “The panel cautioned for use in patients with reactive airway disease…Retrospective studies showed high long-term efficacy of propranolol (57%–81%) when used as a first-line agent for pediatric CVS.155, 156 Two prospective, observational studies in pediatric CVS showed a high response rate to propranolol (77%–93%).157, 158 A larger (n = 81) randomized (uncontrolled and unblinded) trial demonstrated long-term effects of propranolol 1 mg/kg/day on both frequency and severity of CVS attacks with a 92% response rate and superiority over amitriptyline (53% response rate).159“
- Cyproheptadine: “Using criteria of ≥50% improvement in outcomes of interest (episode frequency and duration), 55%–75% (retrospective to randomized) met this threshold. In pediatric migraine, 83% had a positive response.”
- Aprepitant: “The use of aprepitant two or three times per week for prophylaxis resulted in significant improvement in several essential outcomes, including episode frequency, duration, intensity, symptom-free periods, hospitalization rates, and school attendance.69, 169 At the 12-month follow-up, 82% of children [n=95] achieved either partial or complete treatment response.”
- Tricyclic Antidepressants (TCAs): “The panel suggests that this medication be reserved for those with more frequent and severe disease who have not responded to therapies with more favorable side effect profiles. Caution for possible behavioral changes, including suicidality, is indicated in all children and adolescents….Using the common criteria of ≥50% improvement as definition of response (complete or partial), 57% of pediatric and 81% of adult CVS patients responded.”
- Anticonvulsants: “The guideline panel suggests not using anticonvulsants (e.g., topiramate or valproate) for preventing CVS episodes in children and adolescents, except for refractory CVS.”
My take: While data for CVS remains limited, these guidelines are likely to influence how CVS is managed in children.
Related blog posts:
- Cyclic Vomiting ED Protocol
- Abraham Lincoln’s Cyclic Vomiting Action Plan
- Diet or Drugs for Cyclic Vomiting Syndrome This has been one of the most popular posts on this blog and reviewed NASPGHAN CVS guidelines from 2008
- Aprepitant for CVS
- Neuro-Stim for Refractory Cyclic Vomiting?
- Costs/Yield of Diagnosing Cyclic Vomiting Syndrome
- How to Distinguish Cyclic Vomiting Syndrome and Cannabis Hyperemesis Syndrome
- Topiramate -2nd Line Agent for Cyclic Vomiting Syndrome
- Misdirection: False-postive Urine Cannaboid Screen due to Pantoprazole | gutsandgrowth
Disclaimer: This blog, gutsandgrowth, assumes no responsibility for any use or operation of any method, product, instruction, concept or idea contained in the material herein or for any injury or damage to persons or property (whether products liability, negligence or otherwise) resulting from such use or operation. These blog posts are for educational purposes only. Specific dosing of medications (along with potential adverse effects) should be confirmed by prescribing physician. Because of rapid advances in the medical sciences, the gutsandgrowth blog cautions that independent verification should be made of diagnosis and drug dosages. The reader is solely responsible for the conduct of any suggested test or procedure. This content is not a substitute for medical advice, diagnosis or treatment provided by a qualified healthcare provider. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a condition









