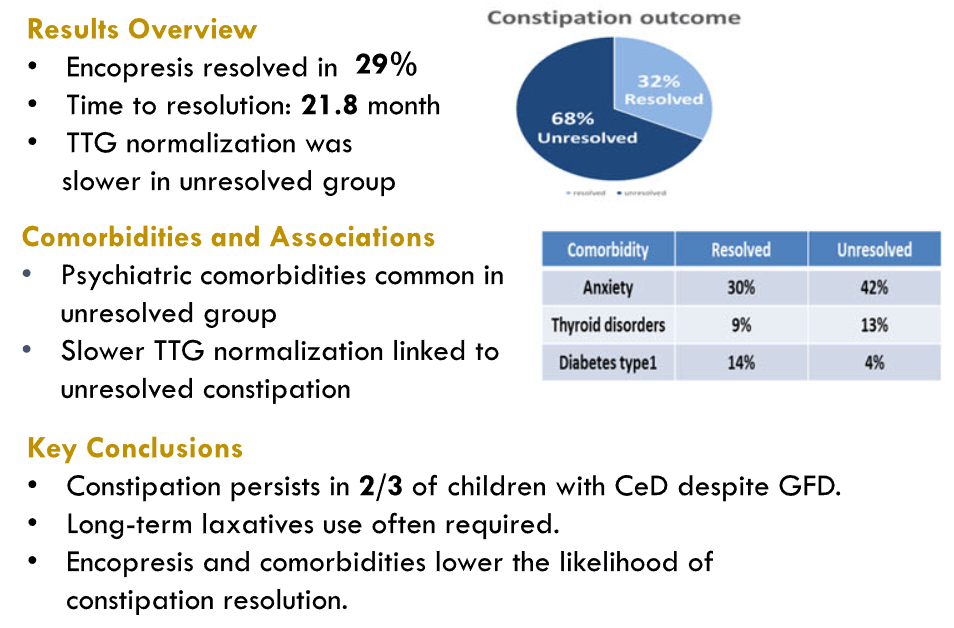
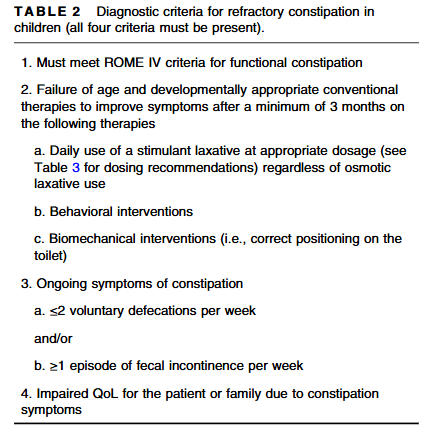
- D Simon et al. J Pediatr Gastroenterol Nutr. 2026;82:407–414. Dolichocolon is common in pediatric gastroenterology patients with constipation and associated complaints
- L Dorfman, A Kaul. J Pediatr Gastroenterol Nutr. 2026;82:320–322 Commentary. Dolichocolon in pediatric patients with constipation—The chicken or the egg?
Methods: In this retrospective study, a total of 155 contrast enemas were administered and then assessed for features of colonic redundancy consistent with dolichocolon (DC), based on a priori imaging (adult) criteria.
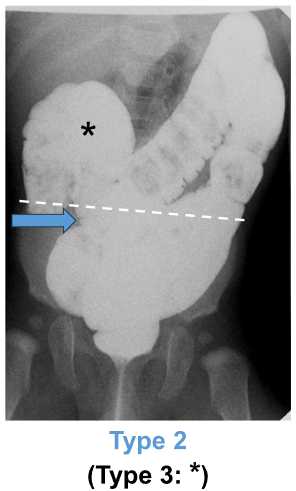
“DC was defined as: any portion of the sigmoid colon reaching above the iliac crest line (Type 1), and/or any portion of the transverse colon reaching below the iliac crestline with or without redundant flexures (Type 2)…We decided not to study Type 3 DC (i.e., redundant loops at the hepatic or splenic flexure, example shown in Figure 1A*) separately because that category was deemed to be arbitrary/imprecise.”
Key findings:
- Consensus‐based identification (i.e., independent agreement among all three reviewers) of dolichocolon (DC) was observed in 74.1% of children under 2 years old and 88.6% of those aged 2–4 years presenting with constipation
- The prevalence subsequently significantly decreased with age, with 68.8% in children aged 5–10 years and 47.6% in adolescents aged 11–17 years. “The pattern of decreasing prevalence of DC with age after 5 years is in contrast to findings in adult patients over 40 years with constipation, where DC frequency was found to increase significantly with age”
- The vast majority (95.6%) of DC was Type 1; 3.5% was Type 2. 0.9% was both Type 1 and Type 2
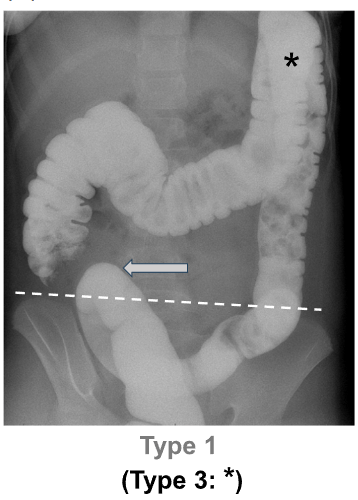
highlights the sigmoid colon reaching above the IC
The editorial by Dorfman et al. notes that “dolichocolon has a long history in medical literature, but its exact role remains uncertain, presenting a classic “chicken or the egg” dilemma…Until more stringent pediatric-specific definitions and longitudinal evidence are acquired, clinicians should exercise caution in solely attributing symptoms to dolichocolon…While dolichocolon may play a role, it is unlikely to be the sole cause.”
My take: I had to read the article because I was not familiar with the term “dolichocolon.” The authors, though, summarize the key point: “the clinical relevance of this radiologic finding is not completely understood.” As a separate matter, a pediatric study on how a dolichocolon affects colonoscopy would be interesting; presumably, it would make it more difficult with longer duration and lower rates of TI intubation.
Related blog posts:
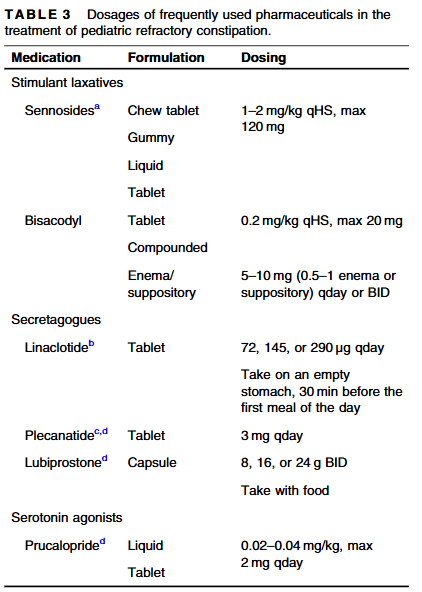
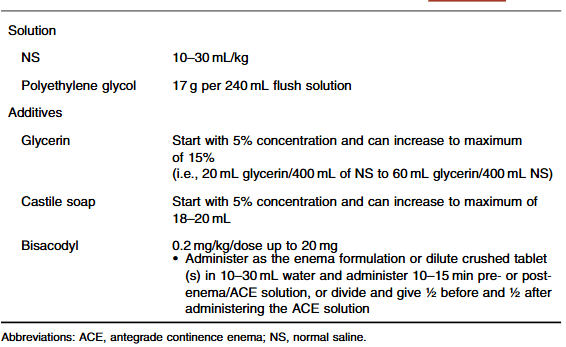
- Position Paper: Pediatric Refractory Constipation Management
- What’s Wrong with Ordering an AXR for Constipation in the ER?
- Radiographs and Constipation -Bad Practice and Good Study
- Our Study: Provider Level Variability in Colonoscopy Yield
- How Procedure Volume Affects Pediatric Colonoscopy Success Rates
- How Much Harder is a Colonoscopy in Children Less Than 6 Years of Age