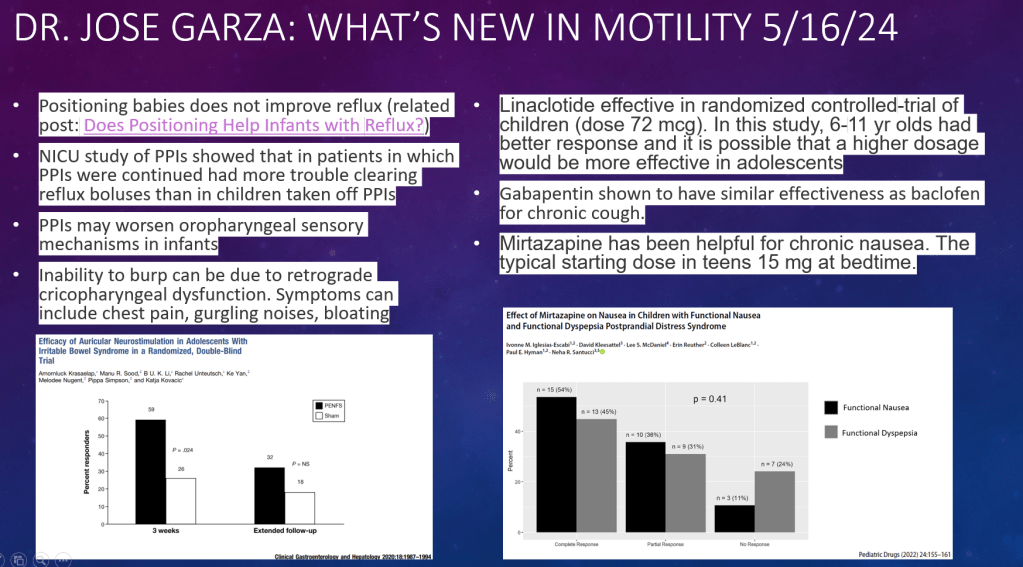
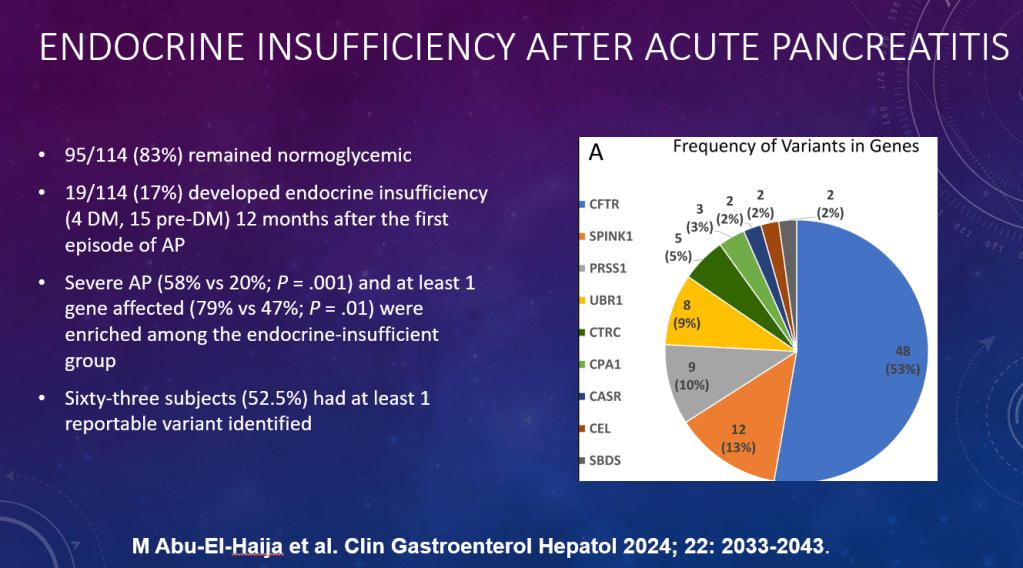
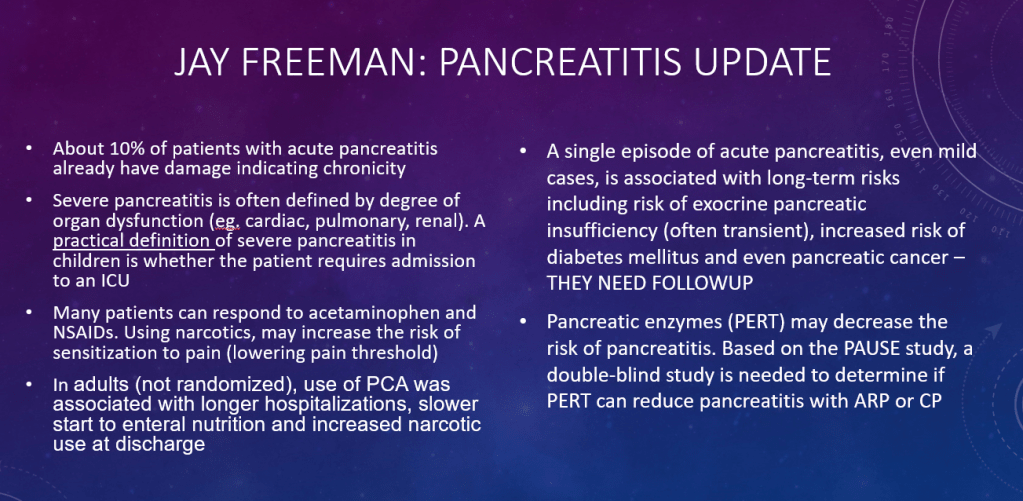
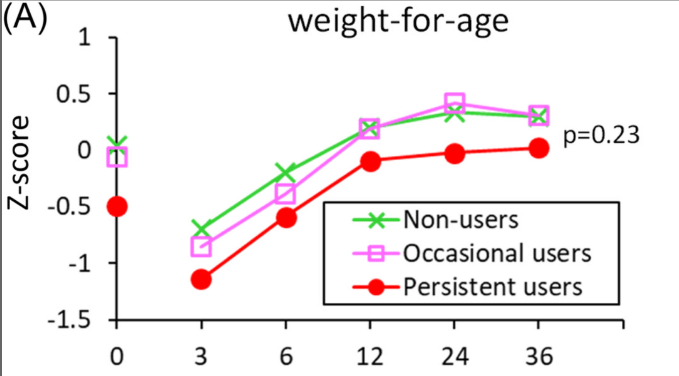
S Diemer et al. JPGN 2025; DOI: 10.1002/jpn3.70050. Open Access! The effect of elexacaftor–tezacaftor–ivacaftor on liver stiffness in children with cystic fibrosis
In this retrospective study, 12 of 21 patients had cystic fibrosis hepato-biliary involvement (CFHBI). The authors examined the liver stiffness after administration of the new and highly potent CF transmembrane conductance regulator modulator therapy, elexacaftor–tezacaftor–ivacaftor (ETI). All of the patients in this cohort had normal liver enzymes.
Key findings:
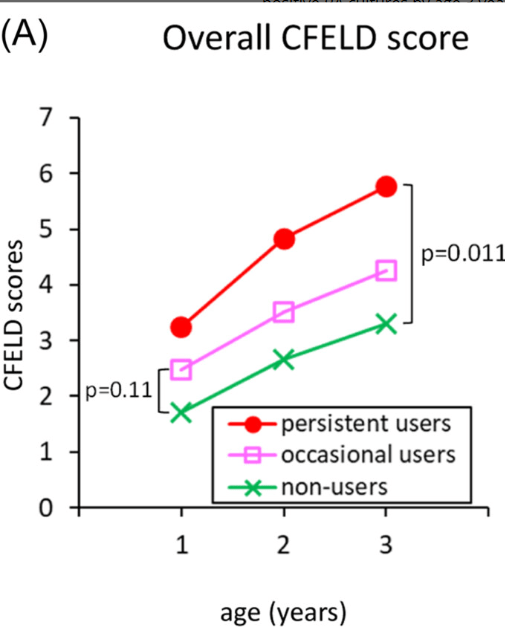
- Analyzing liver stiffness in CwCF with CFHBI showed a decline to 5.7 kPa median (IQR: 3.9–7.1) during ETI treatment, and this decline was statistically significant (W = −60, n = 12, p = 0.0161) (Figure 3B) (after at least 3 months of ETI treatment)

Discussion Points:
“Our findings of a clear improvement of liver stiffness in CwCF and CFHBI during ETI treatment is in line with the recently published study by Terlizzi et al.28 Calvo et al. prospectively investigated liver stiffness and liver enzyme development in a single-centre cohort with a starting point before ETI and a follow-up at 1, 3 and 6 months on ETI…A significant overall reduction in mean liver stiffness was found at 6 months, and already after 1 month of ETI, a decline in liver stiffness was observed in those with values ≥5 kPa.29“
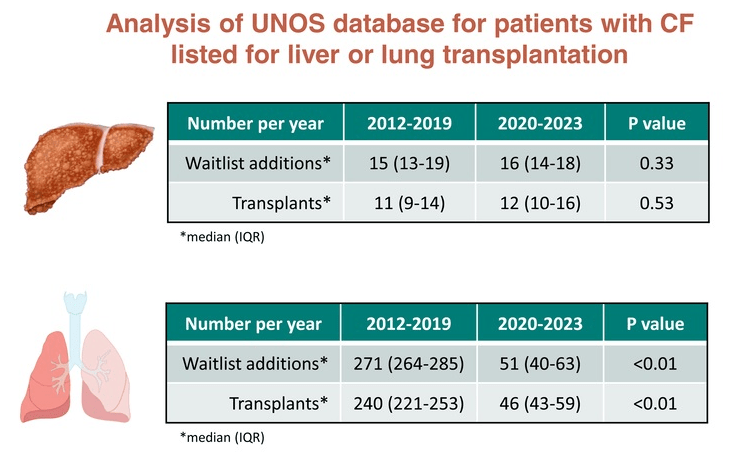
My take: Liver stiffness is a biomarker for chronic liver damage. Longer term studies will be needed to determine how important triple therapy is for liver health in persons with cystic fibrosis. Thus far, there has not been improvement in the number of patients with CF needing a liver transplant; however, there has been a marked improvement in the need for lung transplantation.
Related blog posts:
- Impact of CFTR Modulators on the Need for Liver and Lung Transplantation in Patients with Cystic Fibrosis
- CHOA Nutrition Support Lecture: Cystic Fibrosis Nutrition -Changing in the Age of ‘Miracle Drug’
- Aspen Webinar 2021 Part 7 -Cystic Fibrosis Liver Disease
- Intestinal Inflammation in Patients with Cystic Fibrosis
- Data on Immobilized Lipase Cartridge for Patients with CF
- Big Advance for Cystic Fibrosis -Who Will Benefit?