AJ Yarur et al. Clinical Gastroenterology and Hepatology 2026; 24: 210 – 220. Open Access! Efficacy of Etrasimod in Ulcerative Colitis: Analysis of ELEVATE UC 52 and ELEVATE UC 12 by Baseline Endoscopic Severity.
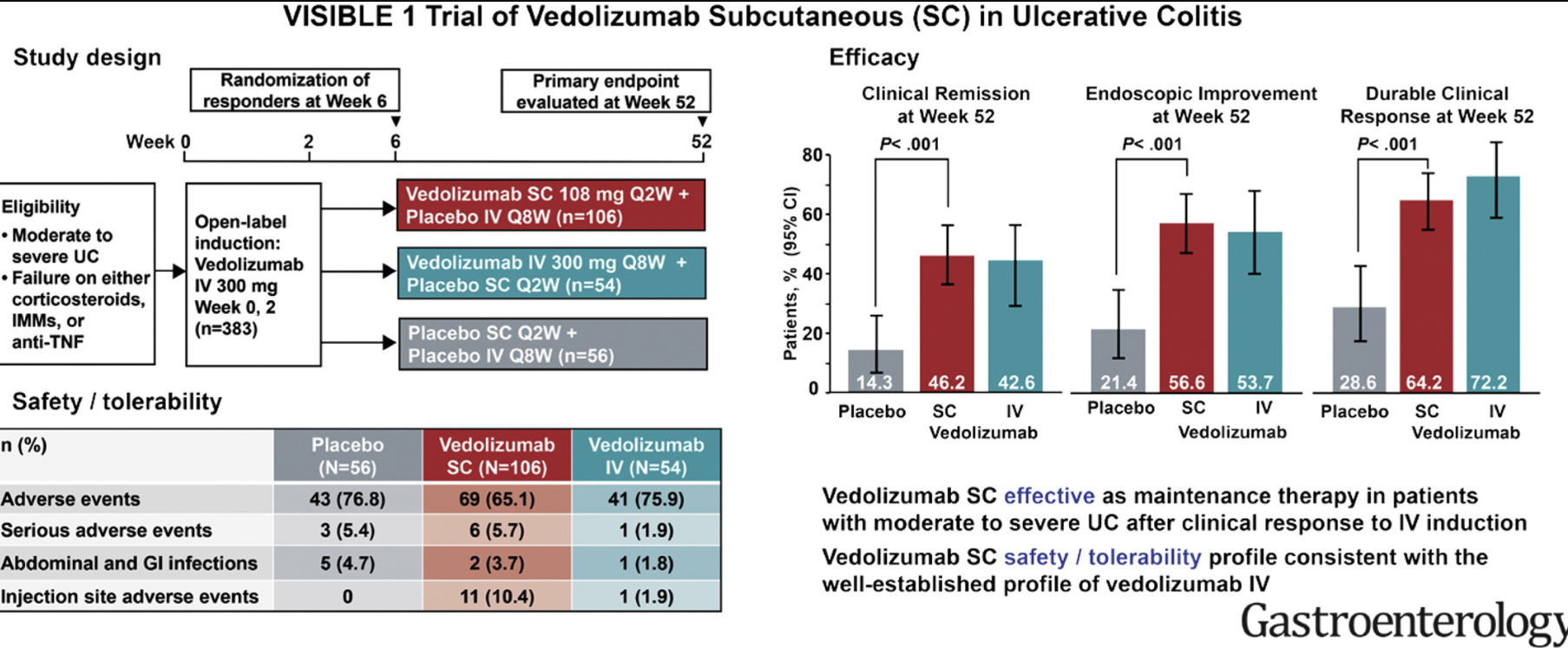
Methods: Efficacy end points were evaluated at Weeks 12 (pooled population) and 52 (ELEVATE UC 52)
Key findings:
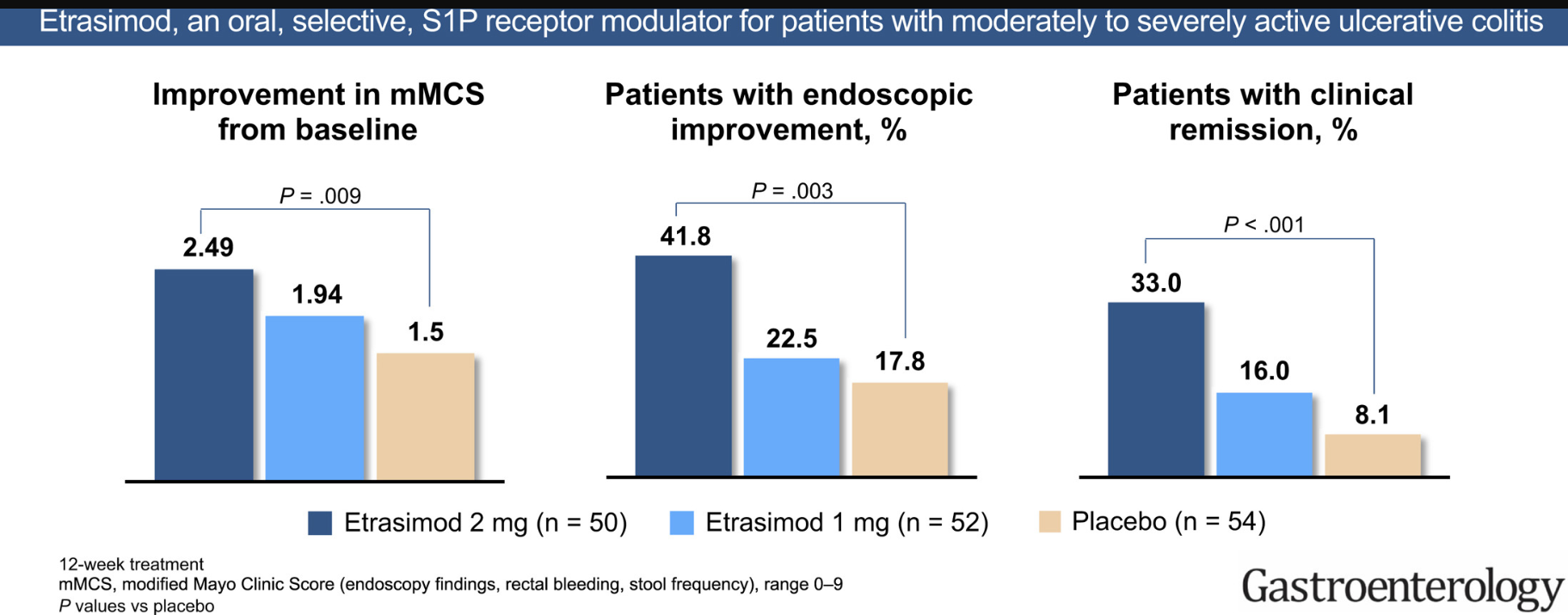
- Clinical remission in the moderate group compared to placebo: Week 12: 38.3% vs 17.9%; Week 52: 36.5% vs 14.3%
- Clinical remission in the severe group compared to placebo: Week 12: 18.2% vs 6.1%; Week 52: 29.4% vs 3.4%
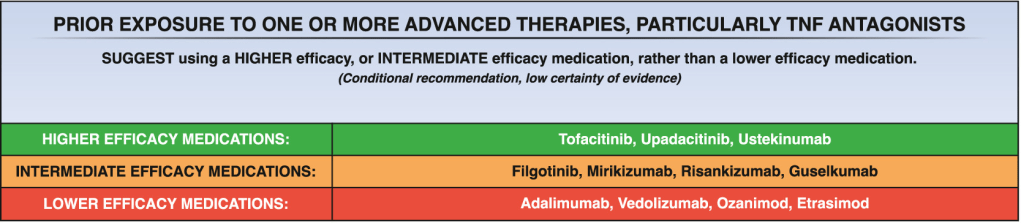
- “Our findings were consistent with those for other UC treatments…with efficacy improvements generally being greater among patients who were naive rather than experienced with biologics and/or JAKi.12–17“

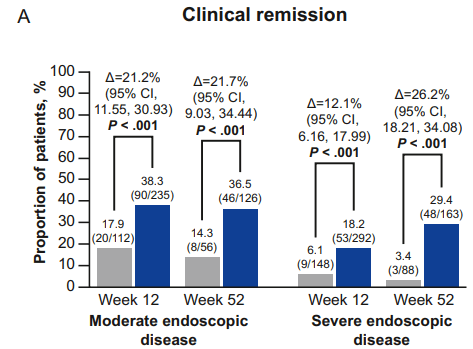
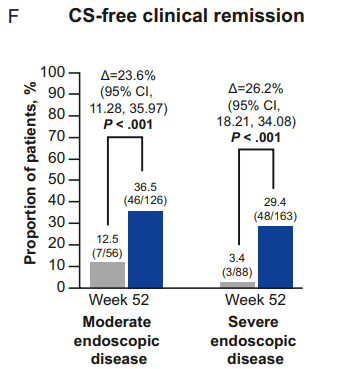
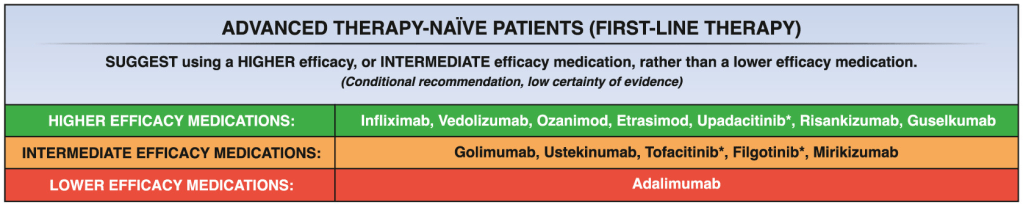
My take: Etrasimod demonstrated significant induction and maintenance efficacy over placebo in both moderate and severe endoscopic disease. Its role remains limited as there are other treatments with improved likelihood of response, especially in those with prior advanced therapies. However, it is notable that recent AGA guidelines promote etrasimod as one of the higher efficacy agents in patients naive to advanced therapies.
Related blog posts: