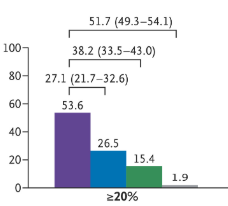NM Al-Roub et al. JAMA Netw Open. 2025;8(12):e2549124. doi:10.1001/jamanetworkopen.2025.49124. Open Access! Body Mass Index and Anthropometric Criteria to Assess Obesity
Background: “Obesity has historically been defined using body mass index (BMI). However, BMI does not account for adipose tissue, limiting its accuracy. The Lancet Diabetes & Endocrinology Commission created a revised obesity definition including anthropometric measures (waist circumference [WC], waist-to-hip ratio [WHR], and waist-to-height ratio [WHtR]),1 encompassing and subcategorizing preclinical obesity (excess adiposity without organ dysfunction or physical impairment) and clinical obesity (a disease).”
Methods: The authors analyzed 14,414 participants representing 237,700,000 US adults. using the 2017-2023 National Health and Nutrition Examination Survey (NHANES)
Key findings:
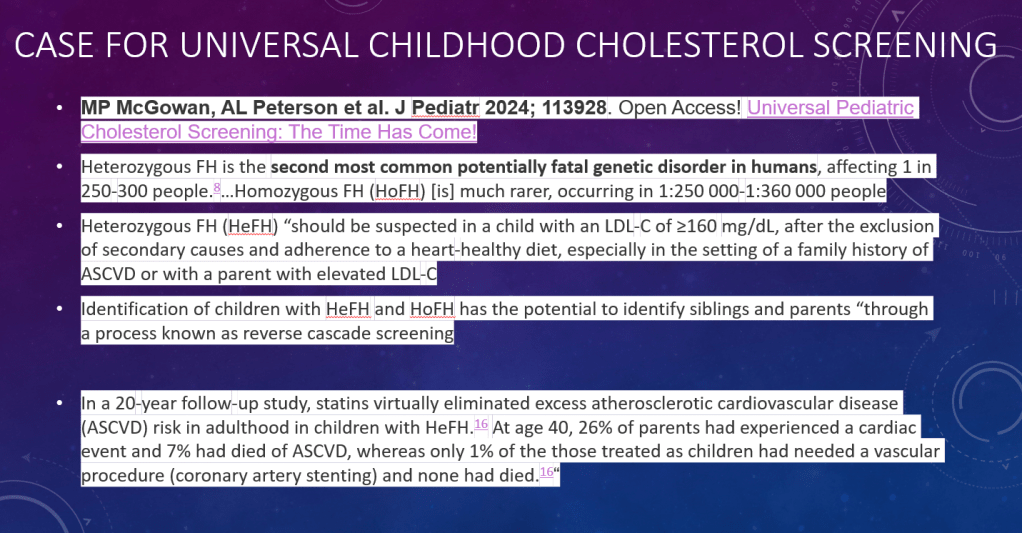
- Survey-weighted obesity prevalence was 75.2%
- Obesity was noted in 100% among adults with BMI of 30 or greater, 80.4% with BMI 25 to less than 30, and 38.5% with BMI less than 25
Discussion Points:
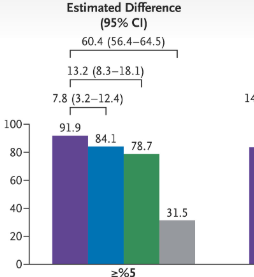
“These findings demonstrate the impact of anthropometric thresholds, particularly since 80.0% of adults had waist-to-height ratio [WHtR]) above 0.5. Though this value was cited by the Lancet Commission and identifies cardiometabolic risk,1,4,5 the commission emphasized that additional research was required for this cutoff.1“
My take: This is a provocative study indicating that even more U.S. adults could be considered obese when incorporating anthropometric criteria. More data is needed to assess the outcomes of this group that is considered obese with new criteria but not by using BMI criteria.
Related blog posts:
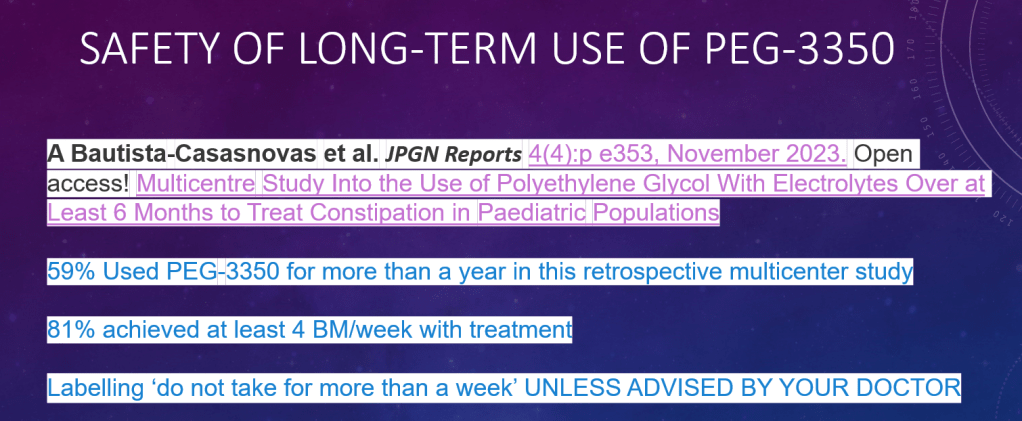
- In the News: Fewer Peanut Allergies, Possibly Improving Obesity Rates in U.S., Best Fruit for Constipation
- Primary Prevention of Obesity Still Needed
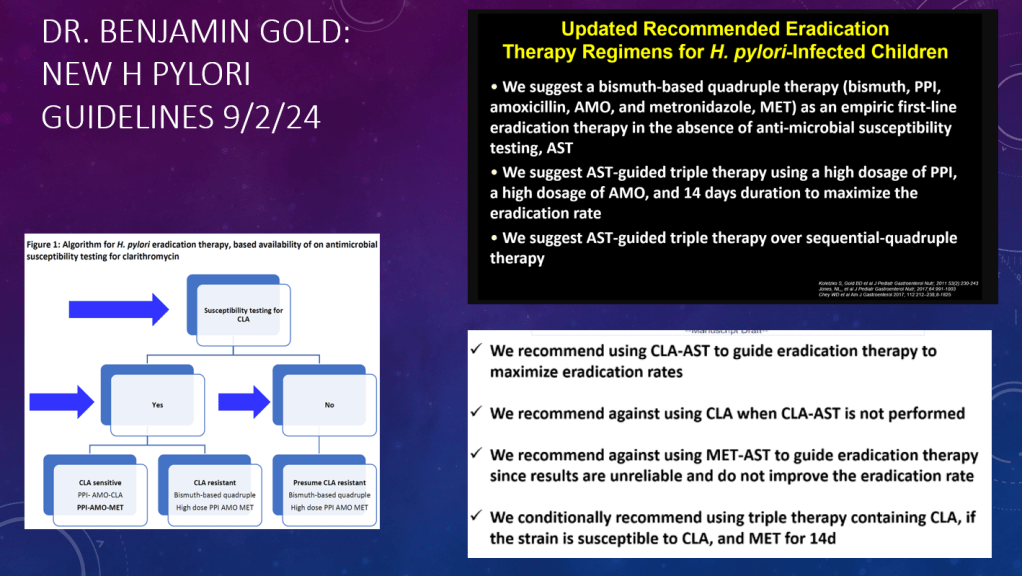
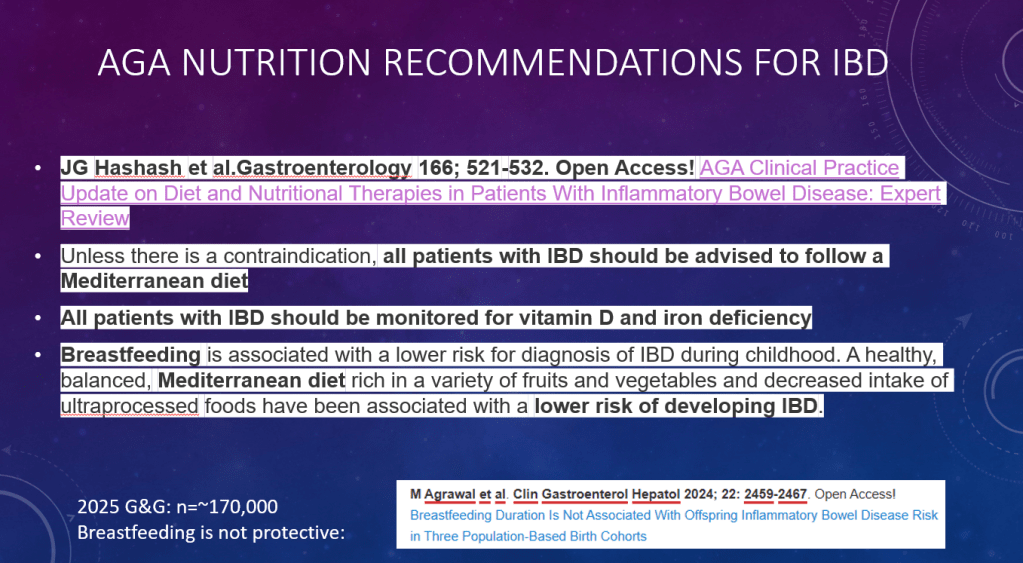
- Diets for Obesity and Steatotic Liver Disease Plus Patient Information from FISPGHAN
- Worldwide Trends in Underweight and Obesity
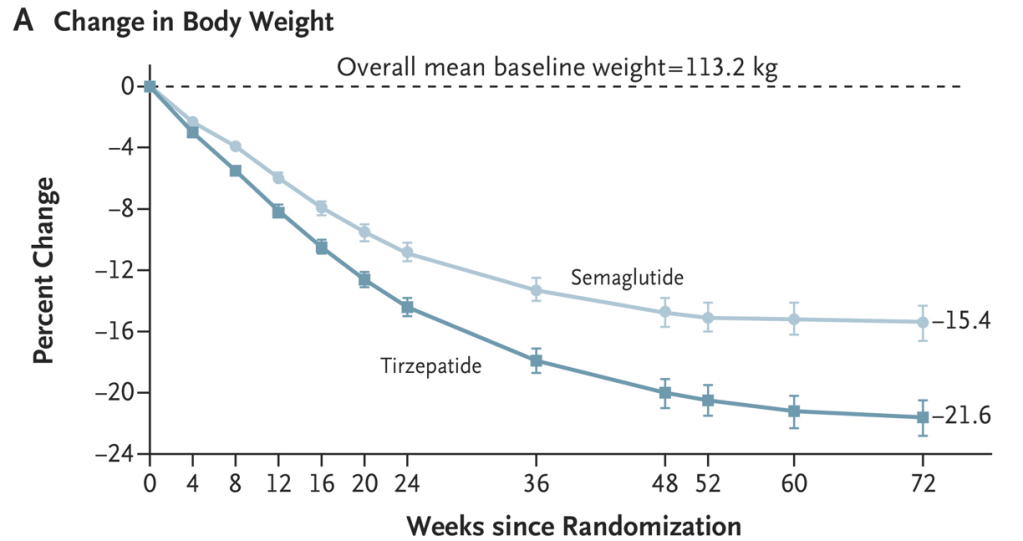
- The Rise of Oral Obesity Therapies: Semaglutide and Orforglipron
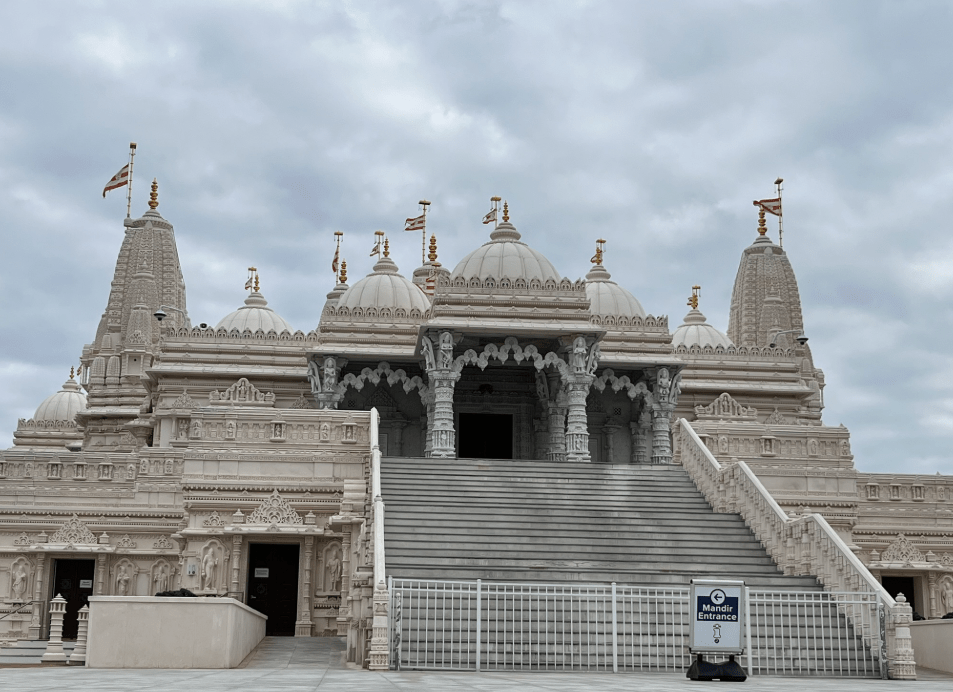
This is a magnificent Hindu spiritual center in Lilburn.
No photos are allowed inside though there are several online (see below).