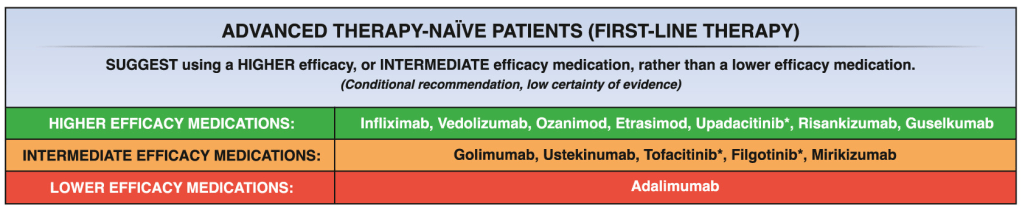
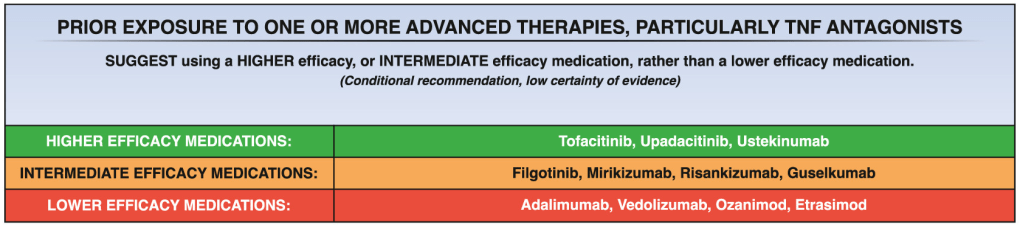
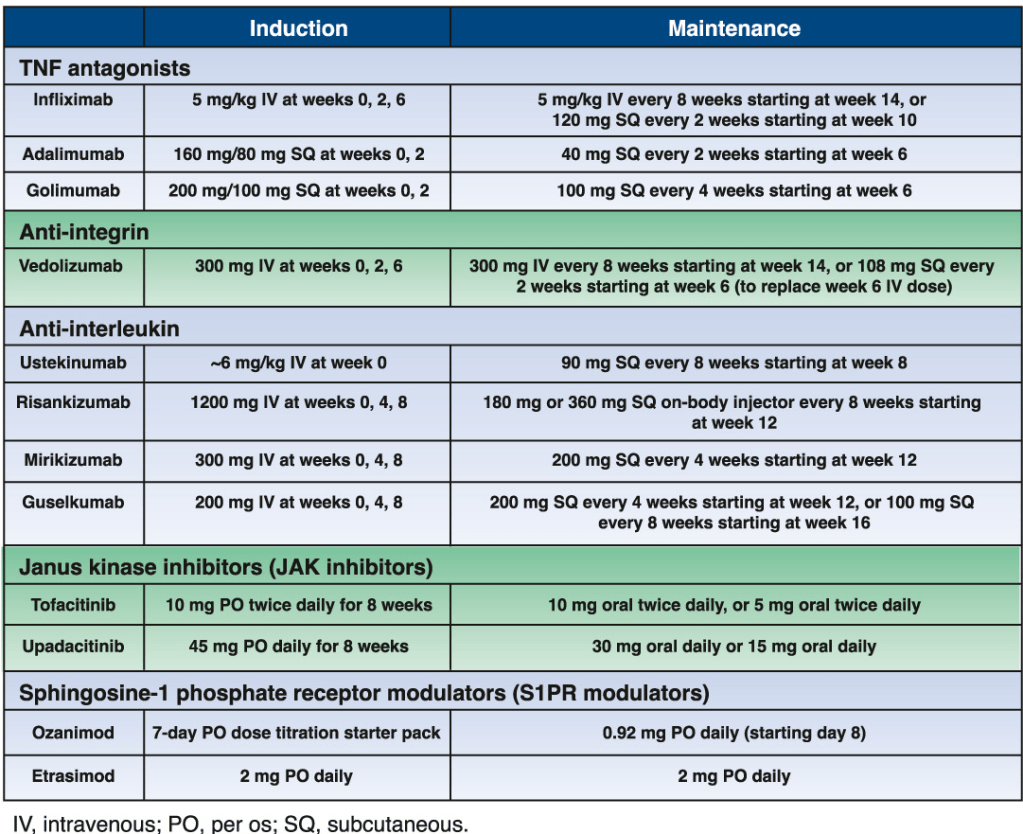
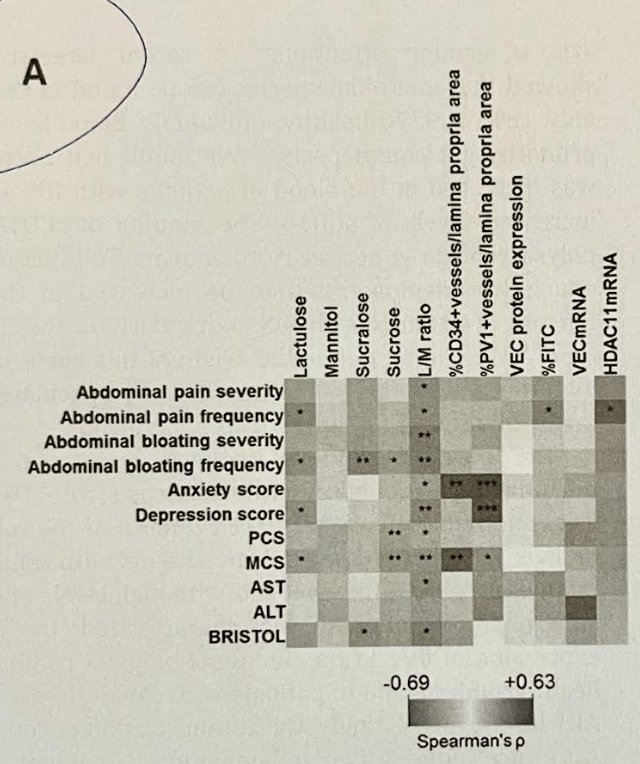
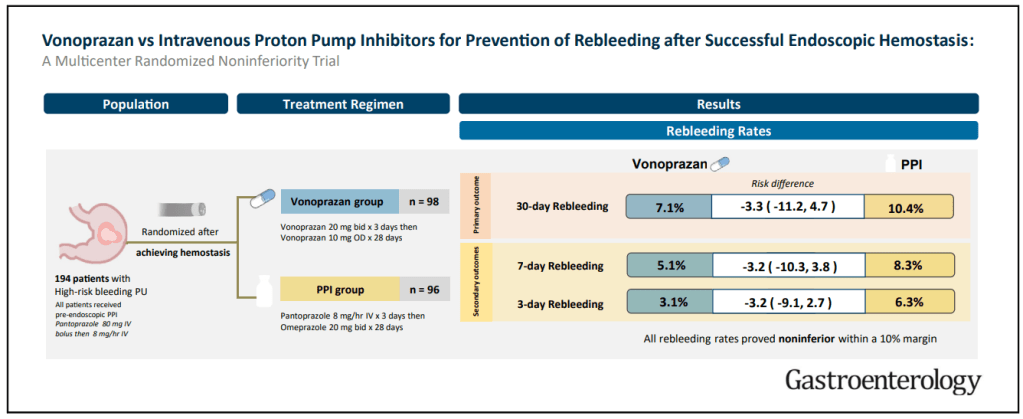
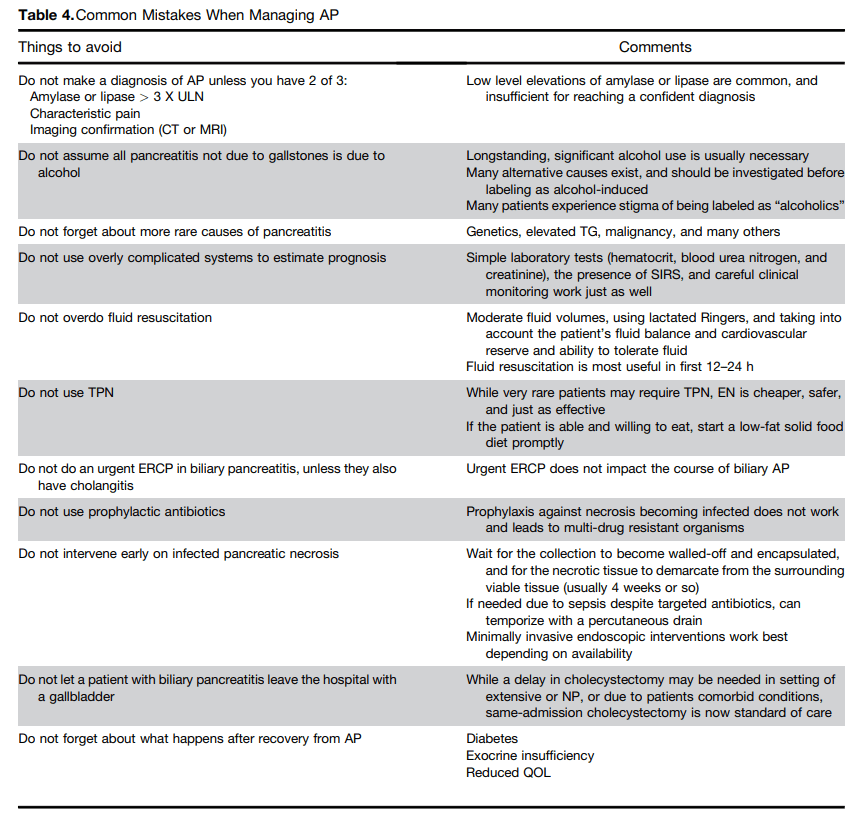
11/18/24 NY Times, G Kolata: Pancreatic Cancer Surge May Be Less Worrisome Than It Seemed (behind paywall)
An excerpt:
One of the first warnings came in a paper published in 2021. There was an unexpected rise in pancreatic cancer among young people in the United States from 2000 to 2018… a new study published on Monday in The Annals of Internal Medicine suggests, the whole alarm could be misguided.
The authors of the paper, led by Dr. Vishal R. Patel, a surgical resident at Brigham and Women’s Hospital in Boston, did not dispute the data showing a rising incidence. They report that from 2001 to 2019 the number of young people — ages 15 to 39 — diagnosed with pancreatic cancer soared. The rate of pancreatic surgeries more than doubled in women and men…
With more pancreatic cancers in young people, there should be more pancreatic cancer deaths. And there were not. Nor were more young people getting diagnosed with later-stage cancers. Instead, the increase was confined to cancers that were in very early stages.
Many cancers will never cause harm if left alone, but with increasingly sensitive tools, doctors are finding more and more of them. Because there usually is no way to know if they are dangerous, doctors tend to treat them aggressively…It’s the hallmark of what researchers call overdiagnosis: a rise in incidence without a linked rise in deaths..
The sudden rise in pancreatic cancer incidence is largely being driven by another type of tumor — endocrine cancers [rather than the more dangerous adenocarcinomas]. They tend to be indolent, taking years or even decades to grow and spread, but occasionally they can turn malignant…
“A lot of patients say, ‘Get it out,’” said Dr. Adewole S. Adamson, an author of the new paper and an overdiagnosis expert at the University of Texas at Austin. “When someone tells you that you have cancer you feel like you have to do something.”
But, said Dr. William Jarnagin, a pancreatic cancer specialist at Memorial Sloan Kettering Cancer Center, removing early stage endocrine tumors “has never been proven to be a good strategy.”
My take: More cases of pancreatic tumors are being detected with the increased use of cross-sectional imaging (eg. CT scan, MRI). It is helpful to know that the increase in (mainly) pancreatic endocrine tumors is not leading to more deaths. Yet, each individual case presents some difficult decisions.
Related blog post:
- Screening for Melanoma in At-Risk (Pediatric) Patients –Is This a Good Idea? In this 2021 NEJM study, there was a 6-fold increase in melanoma diagnosis over 40 years without an increase in mortality.
Disclaimer: This blog, gutsandgrowth, assumes no responsibility for any use or operation of any method, product, instruction, concept or idea contained in the material herein or for any injury or damage to persons or property (whether products liability, negligence or otherwise) resulting from such use or operation. These blog posts are for educational purposes only. Specific dosing of medications (along with potential adverse effects) should be confirmed by prescribing physician. Because of rapid advances in the medical sciences, the gutsandgrowth blog cautions that independent verification should be made of diagnosis and drug dosages. The reader is solely responsible for the conduct of any suggested test or procedure. This content is not a substitute for medical advice, diagnosis or treatment provided by a qualified healthcare provider. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a condition.
From the Museum of Illusions (Atlantic Station, Atlanta):